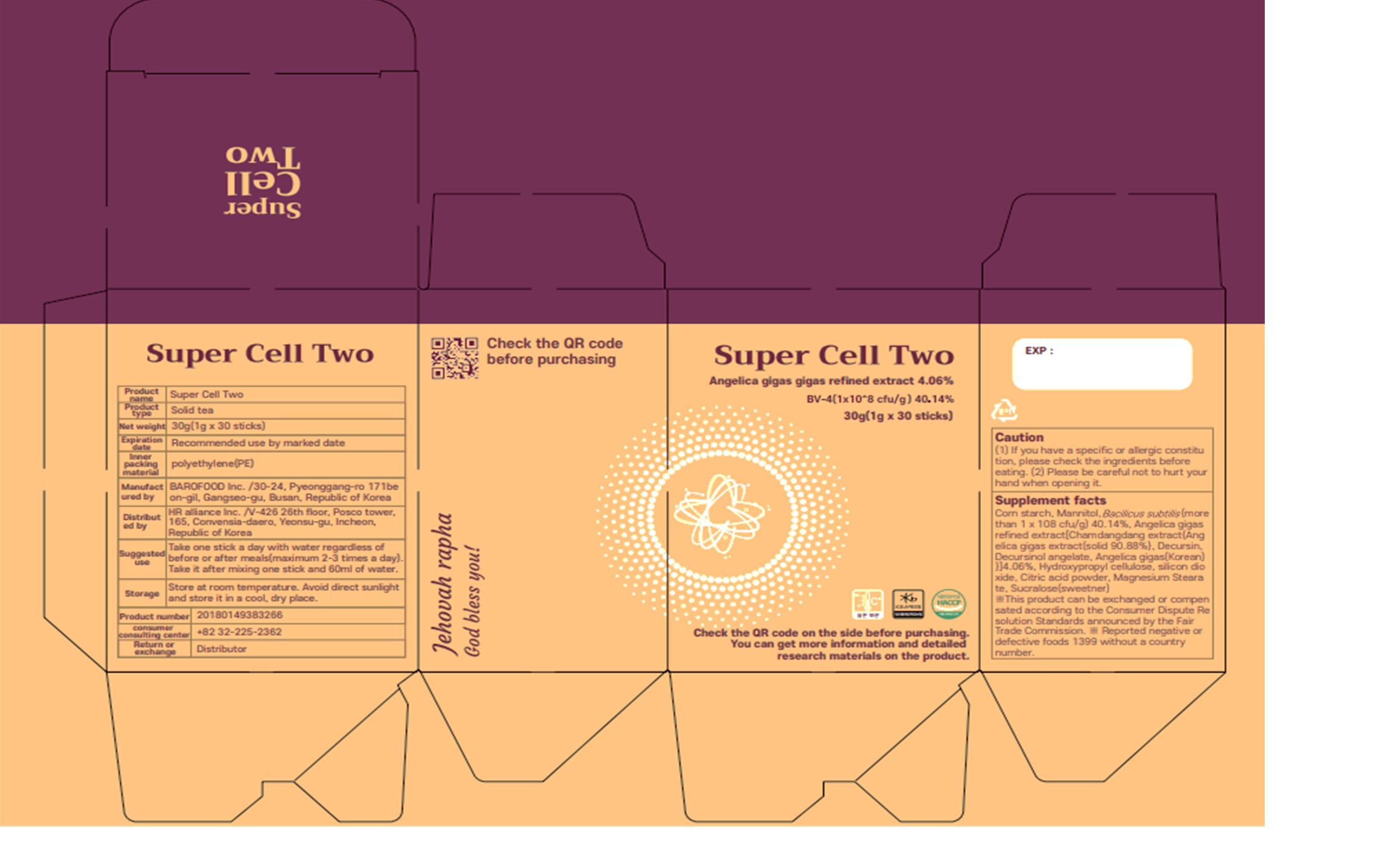
SUPER CELL TWO by BIOHERB Co., Ltd. SUPER CELL TWO
SUPER CELL TWO by
Drug Labeling and Warnings
SUPER CELL TWO by is a Otc medication manufactured, distributed, or labeled by BIOHERB Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SUPER CELL TWO- mannitol, modified corn starch powder
BIOHERB Co., Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
SUPER CELL TWO
Fluids metabolism smooth
Blood metabolism smooth
Reducing body fat
Elimination of waste in blood
Remove swelling
Acquiring gastrointestinal satisfaction
Reducing food intake
These highlights do not include all the information needed to use. See full prescribing information.
Serving Size: 1g * 30EA / Box
1 to 2 times a day, 1 capsuleat a time
Product efficacy and expected treatment effect (no chemicals used)
It has lactic acid bacteria (fermentation) and antiviral (super bacteria, antibacterial action, AIDS, pandemic) treatment effects.
It can treat herpes on the lips and prevent thrombocytopenia, a vascular disorder, and has a complex effect.
| SUPER CELL TWO
mannitol, modified corn starch powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - BIOHERB Co., Ltd. (695625893) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BIOHERB Co., Ltd. | 695625893 | manufacture(77008-1530) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.