SLYND- drospirenone tablet, film coated
SLYND by
Drug Labeling and Warnings
SLYND by is a Prescription medication manufactured, distributed, or labeled by Exeltis USA, Inc., Laboratorios Leon Farma, S.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SLYND safely and effectively. See full prescribing information for SLYND.
SLYND (drospirenone) tablets, for oral use
Initial U.S. Approval: 2001INDICATIONS AND USAGE
SLYND is a progestin indicated for use by females of reproductive potential to prevent pregnancy. (1)
DOSAGE AND ADMINISTRATION
Take one tablet taken daily for 28 days; one white active tablet daily during the first 24 days and one green inactive tablet daily during the 4 following days. (2)
DOSAGE FORMS AND STRENGTHS
SLYND consists of 24 white tablets each containing 4 mg of drospirenone and 4 green inert tablets. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hyperkalemia: Check serum potassium levels during the first treatment cycle in females receiving daily, long-term treatment for chronic conditions of diseases with medications that may increase serum potassium concentrations. (5.1)
- Thromboembolic disorders: Discontinue SLYND if a thromboembolic event occurs. (5.2)
- Bone loss: It is unknown if SLYND may cause a clinically relevant loss of bone mineral density. (5.3)
- Liver Disease: Discontinue use if jaundice or acute or chronic disturbances of liver function develops. (5.5)
- Ectopic pregnancy: Be alert to the possibility of ectopic pregnancy in females who become pregnant or complain of lower abdominal pain while on SLYND. (5.6)
- Risk of Hyperglycemia in Patients with Diabetes: Patients with diabetes may be at greater risk of hyperglycemia and may require additional medication adjustments or monitoring. (5.7)
- Bleeding Irregularities and Amenorrhea: May cause irregular bleeding or amenorrhea. Evaluate for other causes, such as pregnancy, if irregular bleeding or amenorrhea persists. (5.9)
ADVERSE REACTIONS
Most common adverse reactions (>1%) are: acne, metrorrhagia, headache, breast pain, weight increased, dysmenorrhea, nausea, vaginal hemorrhage, libido decreased, breast tenderness, menstruation irregular (6)
To report SUSPECTED ADVERSE REACTIONS, contact Exeltis USA, Inc. at 1-877-324-9349 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Drugs or herbal products that induce certain enzymes (for example, CYP3A4) may decrease the effectiveness of SLYND or increase breakthrough bleeding. Counsel patients to use a back-up or alternative method of contraception when enzyme inducers are used with SLYND. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 How to Use SLYND
2.2 How to Take SLYND
2.3 Missed Doses
2.4 Advice in Case of Gastrointestinal Disturbances
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hyperkalemia
5.2 Thromboembolic Disorders
5.3 Bone Loss
5.4 Cervical Cancer
5.5 Liver Disease
5.6 Ectopic Pregnancy
5.7 Risk of Hyperglycemia in Patients with Diabetes
5.8 Bleeding Irregularities and Amenorrhea
5.9 Depression
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Hormonal Contraceptives
7.2 Influence of SLYND on other Medicinal Products
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 How to Use SLYND
SLYND is dispensed in a blister card. SLYND should be started using a Day 1 start.
Table 1 Instructions for Starting or Switching SLYND Starting SLYND in females with no current use of hormonal contraception (Day 1 Start) Important: Consider the possibility of ovulation and conception prior to initiation of this product.
Tablet Color:
SLYND active tablets are white (Day 1 to Day 24).
SLYND inert tablets are green (Day 25 to Day 28).Day 1 Start:
Take first white active tablet on the first day of menses.
Take subsequent white active tablets once daily at the same time each day for a total of 24 days.
Take one green inert tablet daily for 4 days and at the same time of day that active tablets were taken.
Begin each subsequent pack on the same day of the week as the first cycle pack (i.e., on the day after taking the last inactive tablet).Switching from another contraceptive method to SLYND Start SLYND:
- A Combined Oral Contraceptive (COC)
- On the day when the new pack of the previous COC would have started.
- Transdermal Patch
- On the day when next application would have been scheduled.
- Vaginal ring
- On the day when next insertion would have been scheduled.
- Injection
- On the day when next injection would have been scheduled.
- Intrauterine contraceptive
- On the day of removal
- Implant
- On the day of removal
Refer to the Patient Information and Instructions for Use for additional instructions for counseling patient concerning proper use 2.2 How to Take SLYND
SLYND (white active and green inert tablets) is swallowed whole once a day. Take one tablet daily for 28 consecutive days; one white active tablet daily during the first 24 days and one green inert tablet daily during the 4 following days. Tablets must be taken every day at about the same time of the day so that the interval between two tablets is always 24 hours.
2.3 Missed Doses
Table 2 Instructions for Missed SLYND - If one white active tablet is missed
Take the missed tablet as soon as possible. Continue taking one tablet a day until the pack is finished. - If two or more white active tablets are missed
Take the last missed tablet as soon as possible. Continue one tablet a day until the pack is finished (one or more missed tablet(s) will remain in the blister pack). Additional non-hormonal contraception (such as condoms or spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets. - If one or more green inert tablets are missed
Skip the missed pill days and continue taking one tablet a day until the pack is finished. 2.4 Advice in Case of Gastrointestinal Disturbances
If vomiting or diarrhea occurs within 3-4 hours after tablet taking, the new tablet (scheduled for the next day) should be taken as soon as possible. The new tablet should be taken within 12 hours of the usual time of tablet-taking if possible. If more than two tablets are missed, the advice concerning missed tablets, including using backup non-hormonal contraception, given above is applicable.
-
3 DOSAGE FORMS AND STRENGTHS
SLYND is supplied in blister cards, each containing 24 round, film-coated, unscored, white tablets and 4 round, film-coated, unscored green tablets.
- Each white tablet contains 4 mg of drospirenone. White tablets are debossed with an "E" on one side and a "D" on the other side
- Each green tablet is inert and does not contain drospirenone. Green tablets are debossed with an "E" on one side and a "4" on the other side.
-
4 CONTRAINDICATIONS
SLYND is contraindicated in females with the following conditions:
- Renal impairment [see Warnings and Precautions (5.1) and Use in Specific Populations (8.7)]
- Adrenal insufficiency [see Warnings and Precautions (5.1)]
- Presence or history of cervical cancer or progestin sensitive cancers [see Warnings and Precautions (5.4)]
- Liver tumors, benign or malignant, or hepatic impairment [see Warnings and Precautions (5.5) and Use in Specific Populations (8.6)]
- Undiagnosed abnormal uterine bleeding [see Warnings and Precautions (5.8)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Hyperkalemia
SLYND contains drospirenone, a progestin, which has anti-mineralocorticoid activity, including the potential for hyperkalemia in high-risk females, comparable to a 25 mg dose of spironolactone. SLYND is contraindicated in females with conditions that predispose to hyperkalemia (e.g. renal impairment, hepatic impairment, and adrenal insufficiency). Females receiving daily, long-term treatment for chronic conditions or diseases with medications that may increase serum potassium concentration should have their serum potassium concentration checked prior to starting treatment and during the first treatment cycle. Consider monitoring serum potassium concentration in females at increased risk for hyperkalemia i.e., those females who take a strong CYP3A4 inhibitor long-term and concomitantly with SLYND. Strong CYP3A4 inhibitors include azole antifungals (e.g. ketoconazole, itraconazole, voriconazole), HIV/HCV protease inhibitors (e.g., indinavir, boceprevir), and clarithromycin [see Drug Interactions (7)]. Monitor females taking SLYND who later develop medical conditions and/or begin medication that put them at an increased risk for hyperkalemia.
Most females with hyperkalemia in the clinical development studies of SLYND had mild potassium elevations and/or isolated increases that returned to normal while still on study medication. No concurrent adverse reactions were attributed to hyperkalemia. In the pivotal trial, two females (0.2%) with persistent potassium elevations discontinued SLYND.
5.2 Thromboembolic Disorders
Epidemiological studies have not indicated an association between progestin-only preparations and an increased risk of myocardial infarction, cerebral thromboembolism, or venous thromboembolism.
Combined oral contraceptives containing drospirenone and ethinyl estradiol may be associated with a higher risk of venous thromboembolism (VTE) than those containing some other progestins in combination with ethinyl estradiol. It is unknown whether the risk of VTE is increased with drospirenone alone; however, if there is a risk, it is expected to be lower than that of drospirenone in combination with ethinyl estradiol.
When prescribing SLYND, consider the increased risk of thromboembolism inherent in the postpartum period and in females with a history of thromboembolism
Discontinue SLYND if arterial or venous thromboembolic events occur. Consider discontinuing SLYND, if feasible, in case of SLYND prolonged immobilization due to surgery or illness.
5.3 Bone Loss
Treatment with SLYND leads to decreased estradiol serum levels. It is unknown if this may cause a clinically relevant loss of bone mineral density.
5.4 Cervical Cancer
Some studies suggest that use of combination hormonal contraceptives containing progestin and estradiol has been associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there continues to be controversy about the extent to which such findings may be due to differences in sexual behavior and other factors.
5.5 Liver Disease
Discontinue SLYND if jaundice or acute or chronic disturbances of liver function develop. Do not resume use until markers of liver function return to normal and SLYND causation has been excluded.
SLYND is contraindicated in females with liver tumors, benign or malignant, or hepatic impairment [see Use in Specific Populations (8.6)].
5.6 Ectopic Pregnancy
Be alert to the possibility of ectopic pregnancy in females who become pregnant or complain of lower abdominal pain while on SLYND.
5.7 Risk of Hyperglycemia in Patients with Diabetes
Some patients receiving progestins, including SLYND, may exhibit a decrease in insulin sensitivity. Therefore, patients with diabetes may be at greater risk of hyperglycemia and may require additional medication adjustments or monitoring.
5.8 Bleeding Irregularities and Amenorrhea
Females using SLYND may experience unscheduled (breakthrough or intracyclic) bleeding and spotting, especially during the first three months of use. Bleeding irregularities may resolve over time or by changing to a different contraceptive product. If bleeding persists or occurs after previously regular cycles, evaluate for causes such as pregnancy or malignancy.
Based on subject diaries from four clinical trials of SLYND, 64.4% of females experienced unscheduled bleeding at Cycle 1. This percentage decreased to 40.3% by cycle 13.
A total of 91 out of 2593 subjects (0.4%) discontinued SLYND due to menstrual bleeding disorders including metrorrhagia, menstrual irregular, vaginal hemorrhage, menorrhagia, uterine hemorrhage, and amenorrhea.
If scheduled bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or two active tablets or started taking them on a day later than she should have, consider the possibility of pregnancy at the time of the first missed period and perform appropriate diagnostic measures. If the patient has adhered to the prescribed dosing schedule and misses two consecutive periods, rule out pregnancy.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in other sections of the labeling:
- Hyperkalemia [see Warnings and Precautions (5.1)]
- Bleeding Irregularities and Amenorrhea [see Warnings and Precautions (5.8)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect the exposure of SLYND in females of reproductive potential desiring to prevent pregnancy based on four clinical studies including Study CF111/303 [see Clinical Studies (14)]. The mean time of SLYND exposure ranged from 197 to 328 days. The demographic profile for the pooled study data was: mean age 28 years; mean BMI 25 kg/m2; racial distribution was 83% White; 14% Black; 1% Asian and 2% Other.
Table 3 Adverse Reactions Occurring in ≥ 1% of Females Receiving SLYND in Four Pooled Studies Adverse Reaction Total
N = 2598
n (%)Any adverse reaction 627 (24.1) Acne 98 (3.8) Metrorrhagia 72 (2.8) Headache 71 (2.7) Breast pain 57 (2.2) Weight increased 50 (1.9) Dysmenorrhea 49 (1.9) Nausea 47 (1.8) Vaginal hemorrhage 45 (1.7) Libido decreased 33 (1.3) Breast tenderness 31 (1.2) Menstruation irregular 30 (1.2) -
7 DRUG INTERACTIONS
Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
7.1 Effects of Other Drugs on Hormonal Contraceptives
Substances decreasing the systemic concentrations of hormonal contraceptives (HCs) and potentially diminishing the efficacy of HCs:
Drugs or herbal products that induce certain enzymes, including cytochrome P450 3A4 (CYP3A4), may decrease the systemic concentrations of HCs and potentially diminish the effectiveness of HCs or increase breakthrough bleeding.
Some drugs or herbal products that may decrease the effectiveness of HCs include efavirenz, phenytoin, barbiturates, carbamazepine, bosentan, felbamate, griseofulvin, oxcarbazepine, rifampicin, rifabutin, rufinamide, aprepitant, and products containing St. John's wort.
Interactions between HCs and other drugs may lead to breakthrough bleeding and/or contraceptive failure. Counsel females to use an alternative non-hormonal method of contraception or a back-up method when enzyme inducers are used with HCs, and to continue back-up non-hormonal contraception for 28 days after discontinuing the enzyme inducer to ensure contraceptive reliability.
Substances increasing the systemic concentrations of hormonal contraceptives (HCs):
In a clinical drug-drug interaction study conducted in premenopausal females, once daily co-administration of DRSP 3 mg/ethinyl estradiol (EE) 0.02 mg containing tablets with strong CYP3A4 inhibitor, ketoconazole 200 mg twice daily for 10 days resulted in a moderate increase of DRSP systemic exposure.
7.2 Influence of SLYND on other Medicinal Products
Based on in vitro studies and in vivo interaction studies in female volunteers using omeprazole, simvastatin and midazolam as marker substrate, an interaction of drospirenone with the metabolism of other active substances is unlikely.
Potential to increase serum potassium concentration:
There is a potential for an increase in serum potassium concentration in females taking SLYND with other drugs that may increase serum potassium concentration (for example, ACE inhibitors, angiotensin-II receptor antagonists, potassium-sparing diuretics, potassium supplementation, heparin, aldosterone antagonists, and NSAIDS [see Warnings and Precautions (5.1)]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on epidemiologic studies and meta-analyses, there is little or no increased risk of birth defects in the children of females who inadvertently use oral progestins during early pregnancy (See Data).
Discontinue SLYND if pregnancy occurs, because there is no reason to use hormonal contraceptives during pregnancy
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4 percent and 15 to 20 percent, respectively.
8.2 Lactation
Risk Summary
Negligible amounts of drospirenone are excreted in the breast milk [see Data]. Thus, at therapeutic doses of SLYND, no effects on breastfed newborns/infants are anticipated. In general, no adverse effects have been found on milk production or on the health growth, or development of the infant with use of POPs.
8.4 Pediatric Use
Safety and efficacy of SLYND have been established in females of reproductive age. Safety and efficacy are expected to be the same for postpubertal adolescents under the age of 16 and users 16 years and older.
Study CF111/304 evaluated the bleeding associated with SLYND in females ≥12 years of age. Bleeding data were generally consistent with those from Study CF111/303 in adult females [see Clinical Studies (14)].
Use of this product before menarche is not indicated.
8.5 Geriatric Use
SLYND has not been studied in postmenopausal females and is not indicated in this population.
8.6 Hepatic Impairment
SLYND is contraindicated in females with hepatic impairment [see Contraindications (4), Warnings and Precautions (5.5)]. The mean exposure to drospirenone (DRSP) in females with moderate liver impairment is approximately three times higher than the exposure in females with normal liver function. SLYND has not been studied in females with severe hepatic impairment [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
SLYND is contraindicated in females with renal impairment [see Contraindications (4), Warnings and Precautions (5.1)].
In subjects with creatinine clearance (CLcr) of 50–79 mL/min, serum DRSP levels were comparable to those in a control group with CLcr ≥ 80 mL/min. In subjects with CLcr of 30–49 mL/min, serum DRSP concentrations were on average 37% higher than those in the control group. In addition, there is a potential to develop hyperkalemia in subjects with renal impairment whose serum potassium is in the upper reference range, and who are concomitantly using potassium sparing drugs [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
There have been no reports of serious deleterious effects from overdosage of SLYND. Symptoms that may occur include are nausea, vomiting, and vaginal bleeding. There are no antidotes and treatment should be to provide symptomatic support.
Drospirenone is a spironolactone analogue which has antimineralocorticoid properties. Therefore, serum potassium and sodium, and evidence of metabolic acidosis, should be monitored in cases of overdose.
-
11 DESCRIPTION
SLYND (drospirenone) is for use as an oral contraceptive. It is supplied as clear to a slightly opaque PVC-PVDC/Aluminum blister cards, each holding of 24 white tablets each containing 4 mg of drospirenone, a synthetic progestational compound and 4 green inert tablets.
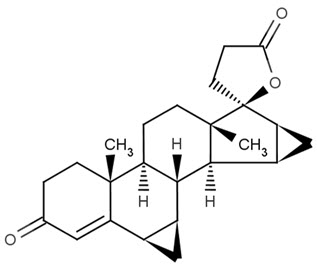
Drospirenone is chemically described as (6R,7R,8R,9S,10R,13S,14S,15S,16S,17S)-1,3',4',6,6a,7,8,9,10,11, 12,13,14,15,15a,16-hexadecahydro10,13-dimethylspiro-[17H-dicyclopropa- [6,7:15,16]cyclopenta[a]phenanthrene-17,2'(5H)-furan]-3,5'(2H)-dione). It has a molecular weight of 366.5, a molecular formula of C24H30O3, and the structural formula below:
Drospirenone is a white to almost white or slightly yellow crystalline powder. It is a progestin and neutral molecule with slight solubility in water
The active tablet is a 5 mm, round, unscored, film-coated, white tablet that contains 4mg of drospirenone as the active ingredient, and microcrystalline cellulose NF, anhydrous lactose NF, colloidal silicon dioxide NF, magnesium stearate NF, polyvinyl alcohol partially hydrolyzed NF, talc NF, titanium dioxide NF, and polyethylene glycol NF as the inactive ingredients. Each tablet is debossed with the letter "E" on one side and the letter "D" on the other sides.
The inert tablet is a 5 mm, round, unscored, film-coated, green tablet that does not contain drospirenone. Each inert green tablet contains the following inactive ingredients: Lactose monohydrate NF, corn starch NF, povidone 30000 NF, colloidal silicon dioxide NF, magnesium stearate NF, hypromellose NF, talc NF, titanium dioxide USP, polysorbate 2910 NF, triacetin NF, FD&C blue 2 aluminum lake and yellow ferric oxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
SLYND progestin-only oral contraceptive lowers the risk of becoming pregnant primarily by suppressing ovulation.
12.2 Pharmacodynamics
Drospirenone is a spironolactone analogue with anti-mineralocorticoid activity.
Laboratory Tests
The use of contraceptive steroids may influence the results of certain laboratory tests, including biochemical parameters of liver, thyroid, adrenal and renal function, serum levels of (carrier) proteins, e.g., corticosteroid binding globulin and lipid/lipoprotein fractions, parameters of carbohydrate metabolism and parameters of coagulation and fibrinolysis.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics of oral drospirenone is dose-proportional following single doses ranging from 1-10 mg. Maximum concentrations (Cmax) of drospirenone in plasma of about 27 ng/ml are reached at about 2-6 hours after single ingestion of SLYND. During a treatment cycle, maximum steady-state concentrations of drospirenone in serum of about 41 ng/ml are reached after about 10 days of treatment. Plasma drospirenone Cmax and area under the curve (AUC) accumulate by a factor of about 1.5 to 2 following multiple dose administration of SLYND. Concomitant ingestion of food has no influence on the extent of absorption of drospirenone.
Distribution
Drospirenone is 95% to 97% bound to serum albumin and does not bind to sex hormone binding globulin (SHBG) or corticosteroid binding globulin (CBG). The apparent volume of distribution of drospirenone is approximately 4 L/kg
Elimination
Metabolism
Drospirenone is extensively metabolized after oral administration. The two main metabolites of DRSP found in human plasma were identified to be the acid form of DRSP generated by opening of the lactone ring and the 4,5-dihydrodrospirenone-3-sulfate, formed by reduction and subsequent sulfation. These metabolites were shown not to be pharmacologically active. Drospirenone is also subject to oxidative metabolism catalyzed by CYP3A4.
Excretion
DRSP serum concentrations are characterized by a terminal disposition phase half-life of approximately 30 hours after both single and multiple dose regimens. Excretion of DRSP was nearly complete after ten days and amounts excreted were slightly higher in feces compared to urine. DRSP was extensively metabolized and only trace amounts of unchanged DRSP were excreted in urine and feces.
Specific Populations
Patients with Hepatic Impairment:
The mean exposure to DRSP in females with moderate liver impairment is approximately three times higher than the exposure in females with normal liver function. SLYND has not been studied in females with severe hepatic impairment [see Contraindications (4) and Warnings and Precautions (5.5)].
Patients with Renal Impairment:
The effect of renal impairment on the pharmacokinetics of DRSP (3 mg daily for 14 days) and the effect of DRSP on serum potassium concentrations were investigated in three separate groups of female subjects (n = 28, age 30–65). All subjects were on a low potassium diet. During the study, 7 subjects continued the use of potassium-sparing drugs for the treatment of their underlying illness. On the 14th day (steady-state) of DRSP treatment, the serum DRSP concentrations in the group with CLcr of 50–79 mL/min were comparable to those in the control group with CLcr ≥ 80 mL/min. The serum DRSP concentrations were on average 37% higher in the group with CLcr of 30–49 mL/min compared to those in the control group. DRSP treatment did not show any clinically significant effect on serum potassium concentration. Although hyperkalemia was not observed in the study, in five of the seven subjects who continued use of potassium-sparing drugs during the study, mean serum potassium concentrations increased by up to 0.33 mEq/L. [see Contraindications (4) and Warnings and Precautions (5.1).]
Drug Interaction Studies:
In a clinical drug-drug interaction study conducted in 20 premenopausal females, co-administration of a product containing DRSP (3 mg)/EE (0.02 mg) COC with the strong CYP3A4 inhibitor ketoconazole (200 mg twice daily) for 10 days increased the AUC(0-24h) and Cmax of DRSP by 2.68-fold (90% CI: 2.44, 2.95) and 1.97-fold (90% CI: 1.79, 2.17), respectively.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month oral carcinogenicity study in mice with doses up to 10 mg/kg/day DRSP, equating to 2 times the maximum clinical exposure (based on AUC), there was an increase in carcinomas of the harderian gland in the high dose DRSP group. In a similar study in rats given doses up to 10 mg/kg/day DRSP, 10 times the maximum clinical exposure (based on AUC), there was an increased incidence of benign and total (benign and malignant) adrenal gland pheochromocytomas in the high dose DRSP group. Mutagenesis studies for DRSP were conducted in vivo and in vitro and no evidence of mutagenic activity was observed.
-
14 CLINICAL STUDIES
Pregnancy Prevention
The efficacy of SLYND was evaluated in Study CF111/303 (NCT02269241). This single arm multicenter, clinical trial was conducted in the U.S. The efficacy population consisted of 953 females ≤ 35 years of age with 5,547 evaluable cycles. The demographic profile for females was: mean age 26.4 years and mean BMI 28.5 kg/m2. The racial distribution was 53.3% Caucasian; 38.5% African American; 2.2% Asian and 6% other. During these cycles, a total of 17 (1.8%) females reported pregnancy, leading to a Pearl Index (95% CI) of 4.0 (2.3, 6.4).
One female who became pregnant during the study was breastfeeding and not included in the Pearl Index (PI) calculation. The confidence interval for the PI was calculated assuming that events of pregnancy had a Poisson distribution.
Out of the 953 females evaluated for efficacy, 332 subjects had a baseline BMI ≥ 30 (35%) and 173 females had a baseline BMI ≥ 35 (18%). Data were insufficient to analyze PI by BMI subgroups.
Table 4 Pearl Index Based on Evaluable Cycles and Reported Pregnancies in Females ≤ 35 Years of Age in Study CF111/303 SLYND
(N = 953)Subjects with pregnancy, n (%) 17 (1.8) Subjects without pregnancy, n (%) 936 (98.2) Total number of evaluable cycles 5547 Pearl Index for evaluable cycles 4.0 95% Confidence Interval for Pearl Index, Lower Limit, Upper Limit 2.3, 6.4 Effect on Bleeding Patterns
The bleeding pattern with SLYND was assessed systematically using patient diaries in Study CF111/303 in adult females.
The percentage of females experiencing scheduled bleeding or unscheduled bleeding/spotting decreased over time. Overall, the percentage of females with scheduled bleeding or spotting decreased from 81% in Cycle 1 to 26% in Cycle 13. Similarly, the overall percentage of females with unscheduled bleeding or spotting decreased from 61% in Cycle 1 to 40% in Cycle 13. The percentages of females with scheduled and unscheduled bleeding or spotting generally decreased through Cycle 10 and were maintained at a consistent level thereafter.
Table 5 Adult Females with Scheduled and Unscheduled Bleeding or Spotting: (Safety Set) Scheduled Unscheduled Cycle n/m* Rate and 95% CI (%) n/m* Rate and 95% CI (%) - * Abbreviations: m = number of subjects with cycle data; n = number of subjects with bleeding or spotting.
Cycle 1 1768/2178 81.2 (79.5, 82.8) 1337/2178 61.4 (59.3, 63.4) Cycle 6 507/1482 34.2 (31.8, 36.6) 703/1482 47.4 (44.9, 50.0) Cycle 13 185/700 26.4 (23.2, 29.7) 282/700 40.3 (36.7, 43.9) In Study CF111/304 conducted in Europe in post-menarchal, female, adolescents (12 through 17 years of age), bleeding data were generally consistent with those from Study CF111/303 in adult females. SLYND was associated with a decrease in the percentage of adolescent females experiencing bleeding or spotting over time. The percentage of adolescent females with scheduled bleeding or spotting decreased from 98.0% in Cycle 1 to 28.4% in Cycle 13. The percentage of adolescent females with scheduled bleeding or spotting generally decreased through Cycle 9 and was maintained at a consistent level thereafter. In contrast, the percentage of adolescent females with unscheduled bleeding or spotting was maintained at a relatively consistent level during the study (53.0% in Cycle 1 versus 52.2% in Cycle 13).
In addition to Studies CF111/303 and CF111/304 an additional two studies evaluated bleeding associated with SLYND. A total of 91 females (0.4%) from these four studies discontinued SLYND due to problems with irregular bleeding or amenorrhea.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
SLYND (drospirenone) tablets is packaged in clear to a slightly opaque PVC-PVDC/Aluminum blister cards. Each blister card holds 24 white round active film-coated tablets, each containing 4mg of drospirenone and 4 green round inert film-coated tablets that do not contain drospirenone. SLYND is supplied in cardboard cartons containing 1, 3, or 6 blister cards as provided below:
SLYND 1 blister card (1 × 28 tablets) NDC: 0642-7470-01
SLYND 3 blister cards (3 × 28 tablets) NDC: 0642-7470-03
SLYND 6 blister cards (6 × 28 tablets) NDC: 0642-7470-06
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved Patient Labeling (Patient Information and Instructions for Use).
SLYND and Dosing Instructions
Advise females on proper daily use of SLYND and what to do if she misses a pill [see Dosage and Administration (2) and FDA-approved Patient Information]. Inform patient that amenorrhea may occur and instruct patient to check for pregnancy if she misses two consecutive periods.
Counsel patients on the following information:
Recommendation to check serum potassium levels during the first treatment cycle in females receiving daily, long-term treatment for chronic conditions of diseases with medications that may increase serum potassium concentrations
Sexually Transmitted Infections
SLYND does not protect against HIV-infection (AIDS) and other sexually transmitted infections.
Use during Pregnancy
SLYND is not to be used during pregnancy; instruct the patient to stop use if pregnancy is confirmed during treatment [see Use in Specific Populations (8.1)].
Drug Interactions
Advise females to inform their healthcare provider if they take herbal supplements such as St. John's wort [see Drug Interactions (7.1)].
- SPL UNCLASSIFIED SECTION
-
PATIENT INFORMATION SLYND (slind) (drospirenone) tablets, for oral use
Progestin pills help to lower the chance of becoming pregnant when taken as directed. They do not protect against HIV infection (AIDS) and other sexually transmitted diseases (STDs).
What is SLYND?
SLYND is a birth control pill (oral contraceptive) also called a POP (progestin only pill) that is used by females who can become pregnant to prevent pregnancy.
The progestin drospirenone may increase potassium levels in your blood. You should not take SLYND if you have kidney, liver or adrenal disease because this could cause serious heart problems as well as other health problems. Other medicines may also increase potassium levels in your blood. If you are currently on daily, long-term treatment for a chronic health condition with any of the medicines listed below, talk to your healthcare provider about whether SLYND is right for you. If you take any of the medicines listed below for a chronic health condition you should have a blood test to check the potassium level in your blood before you start taking SLYND and during the first month that you take SLYND.
- medicines to treat fungal infections, such as ketoconazole, itraconazole, or voriconazole
- medicines to treat Human Immunodeficiency Virus (HIV) infection or Hepatitis C infection, such as indinavir or boceprevir
- clarithromycin
How does SLYND work for contraception?
Your chance of getting pregnant depends on how well you follow the directions for taking your birth control pills. The better you follow the directions, the less chance you have of getting pregnant.
Based on the results of one clinical study of a 28-day regimen of SLYND about 4 out of 100 females may get pregnant within the first year they use SLYND.
The following chart shows the chance of getting pregnant for females who use different methods of birth control. Each box on the chart contains a list of birth control methods that are similar in effectiveness. The most effective methods are at the top of the chart. The box on the bottom of the chart shows the chance of getting pregnant for females who do not use birth control and are trying to get pregnant.
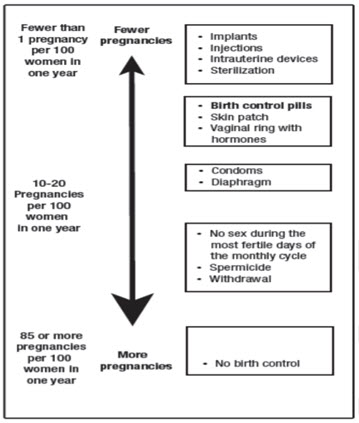
Do not take SLYND if you:
- have kidney disease or kidney failure.
- have reduced adrenal gland function (adrenal insufficiency).
- have or have had cervical cancer or any cancer that is sensitive to female hormones.
- have liver disease, including liver tumors.
- have unexplained vaginal bleeding.
Tell your healthcare provider if you have or have had any of these conditions. Your healthcare provider can suggest a different method of birth control.
If any of these conditions happen while you are taking SLYND, stop taking SLYND right away and talk to your healthcare provider. Use non-hormonal contraception when you stop taking SLYND.
Before you take SLYND, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or think you may be pregnant.
- have ever had blood clots in your legs (deep vein thrombosis), lungs (pulmonary embolism) or a stroke or heart attack (myocardial infarction).
- have or have had depression
Tell your healthcare provider about all the medicine you take including prescription and over-the-counter medicines, vitamins and herbal supplements, such as St. John's Wort.
SLYND may affect the way other medicines work, and other medicines may affect how well SLYND works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take SLYND?
Read the detailed Instructions for Use at the end of this Patient Information leaflet about the right way to take SLYND.
What are the possible serious side effects of SLYND?
SLYND may cause serious side effects, including:
- -
High potassium levels in your blood (hyperkalemia). Certain medicines and conditions can also increase the potassium levels in your blood. Your healthcare provider may check the potassium levels in your blood before and during treatment with SLYND. Call you healthcare provider or go to a hospital emergency room right away if you have signs or symptoms of high potassium levels in your blood including:
- weakness or numbness in an arm or leg
- palpitations (feel like your heart is racing or fluttering) or irregular heartbeat
- nausea
- vomiting
- severe pain in your chest
- shortness of breath.
- -
Blood clot forming in blood vessels (thromboembolism problems). Tell your healthcare provider if you have had a blood clot. Tell your healthcare provider if you plan to have surgery or are not able to be active due to illness or injury. Call your healthcare provider or go to a hospital emergency room right away if you have:
- leg pain that will not go away
- a sudden, severe headache unlike your usual headaches
- sudden, severe shortness of breath
- sudden change in vision or blindness
- chest pain
- weakness or numbness in your arm or leg
- trouble speaking
- - Bone loss. It is not known if the decrease in a sex hormone that happens with SLYND can result in decreased bone density (bone loss).
- - Cervical cancer. See "Do birth control pills cause cancer?"
- - Liver problems, including rare liver tumors. Call your healthcare provider right away if you have yellowing of your skin or eyes.
- - Ectopic pregnancy (pregnancy in your tubes). If you get pregnant while using SLYND, you might have an ectopic pregnancy. That means that the pregnancy is not in the uterus. Ectopic pregnancy is a medical emergency that often requires surgery. If you have severe abdominal (belly) pain, call your healthcare provider or go to a hospital emergency room right away.
- - Risk of high blood sugar levels in people with diabetes. If you have diabetes, you may need to monitor your blood sugar level more often or adjust your diabetes medicine.
- - Changes in menstrual bleeding. Irregular vaginal bleeding, especially between menstrual periods, and irregular periods or the absence of menstrual periods are common side effects of SLYND, but can sometimes be serious. Tell your healthcare provider if you have any of these changes in menstrual bleeding.
- - Depression, especially if you have had depression in the past. Call your healthcare provider immediately if you have any thoughts of harming yourself.
What are the most common side effects of SLYND?
The most common side effects of SLYND include:
- acne
- headache
- breast pain and tenderness
- weight gain
- menstrual cramps
- nausea
- severe vaginal bleeding
- less sexual desire
These are not all the possible side effects of SLYND.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What else should I know about taking SLYND?
- If you are scheduled for any lab tests, tell your healthcare provider you are taking SLYND. Certain blood tests may be affected by SLYND.
How should I store SLYND?
- Store SLYND at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep SLYND and all medicines out of the reach of children.
General information about the safe and effective use of SLYND.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use SLYND for a condition for which it was not prescribed. Do not give SLYND to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about SLYND that is written for health professionals.
Do birth control pills cause cancer?
Hormonal contraceptives do not appear to cause breast cancer. However if you have breast cancer now, have had it in the past, or you have (or have had) another cancer that may be sensitive to hormones, do not use hormonal contraceptives.
Women who use hormonal contraceptives may have a higher chance of getting cervical cancer. However, this may be due to other reasons such as having more sexual partners and exposure to the human papilloma virus (HPV).
What if I want to become pregnant?
You may stop taking SLYND whenever you wish. Consider a visit with your healthcare provider for a pre-pregnancy checkup before you stop taking SLYND.
What should I know about my period when taking SLYND?
Some females may miss a period. Irregular vaginal bleeding or spotting may happen while you are taking SLYND, especially during the first few months of use. If the irregular vaginal bleeding or spotting continues or happens again after you have had regular menstrual cycles call your healthcare provider. It is important to continue taking your pills on a regular schedule to prevent a pregnancy.
What if I miss my scheduled period when using SLYND?
Some females miss periods on hormonal birth control, even when they are not pregnant. However, if you go 2 or more months in a row without a period, or you miss your period after a month where you did not use all of your SLYND correctly, call you healthcare provider because you may be pregnant. Also call your healthcare provider if you have symptoms of pregnancy such as morning sickness or unusual breast tenderness. Stop taking SLYND if you are pregnant.
What are the ingredients in SLYND?
White tablets
Active ingredient: drospirenone
Inactive ingredients: microcrystalline cellulose, anhydrous lactose, colloidal silicon dioxide, magnesium stearate, polyvinyl alcohol partially hydrolyzed, talc, titanium dioxide, and polyethylene glycol.
Green tablets
Inactive ingredients: lactose monohydrate, corn starch, povidone 30000, colloidal silicon dioxide, magnesium stearate, hypromellose, talc, titanium dioxide, polysorbate 2910, triacetin, FD&C blue 2 aluminum lake and yellow ferric oxide.
-
Instructions For Use SLYND (slind) (drospirenone) tablets, for oral use
Important Information about taking SLYND
Before you start taking SLYND
- Decide what time of day you want to take your pill. It is important to take it at the same time every day and in the order as directed on your blister pack.
- Have backup contraception (condoms or spermicide) available.
How to take SLYND
- Take 1 pill every day at the same time. Take the pills in the order directed on your blister pack.
- Both the white pills and the green pills should be swallowed whole.
- Do not skip your pills, even if you do not have sex often. If you miss pills (including starting the blister pack late) you could get pregnant. The more pills you miss, the more likely you are to get pregnant.
- If you have trouble remembering to take SLYND, talk to your healthcare provider. When you first start taking SLYND, spotting or light bleeding in between your periods may occur. Contact your healthcare provider if this does not go away after a few months.
- You may feel sick to your stomach (nauseous), especially during the first few months of taking SLYND. If you feel sick to your stomach, do not stop taking the pill. The problem will usually go away. If your nausea does not go away, call your healthcare provider.
- Missing pills can also cause spotting or light bleeding, even when you take the missed pills later. On the days you take 2 pills to make up for missed pills (see below), you could also feel a little sick to your stomach.
- Some females miss periods on hormonal birth control, even when they are not pregnant. However, if you miss a period and have not taken SLYND according to directions, or miss 2 periods in a row, or feel like you may be pregnant, call your healthcare provider. If you have a positive pregnancy test, you should stop taking SLYND.
- If you have vomiting or diarrhea within 3 to 4 hours of taking your pill, take a new pill (the pill scheduled for the next day) from your blister pack within 12 hours of the usual time you take your pill, if possible. Continue taking all your remaining pills in order. Start the first pill of your next blister pack the day after finishing your current blister pack. This will be 1 day earlier than originally scheduled. Continue on your new schedule.
- If you have vomiting or diarrhea for more than 1 day, your birth control pills may not work as well. If you have sex within 7 days after 1 or more days of vomiting or having diarrhea, use an additional form of birth control, like condoms or spermicide, as back-up contraception.
When should I start taking SLYND?
If you start taking SLYND and you are not currently using a hormonal birth control method:
- Start SLYND on the first day (Day 1) of your natural menstrual period (Day 1 Start). Your healthcare provider should tell you when to start taking your birth control pill.
If you start taking SLYND and you are switching from another birth control pill:
- Start your new SLYND blister pack on the same day that you would start the next pack of your previous birth control method.
- Do not continue taking the pills from your previous birth control pack.
If you start taking SLYND and you are switching from a vaginal ring or transdermal patch:
- Start taking SLYND on the day you would have inserted the next ring or applied the next patch.
If you start taking SLYND and you are switching from a progestin-only method such as an implant or injection:
- Start taking SLYND on the day of removal of your implant or on the day when you would have had your next injection.
If you start taking SLYND and you are switching from an intrauterine device or system (IUD or IUS):
- Start taking SLYND on the day of removal of your IUD or IUS.
Keep a calendar to track your period:
SLYND Day 1 Start:
You will use a Day 1 Start if your healthcare provider told you to take your first pill (Day 1) on the first day of your period.
- Take 1 pill every day in the order of the blister pack, at the same time each day, for 28 days.
- After taking the last pill on Day 28 from the blister pack, start taking the first pill from a new pack, on the same day of the week as the first pack. Take the first pill in the new pack whether or not you are having your period.
Instructions for using your pill blister pack:
Step 1. Look at your SLYND pill pack. See Figure A.
The SLYND pill pack has:
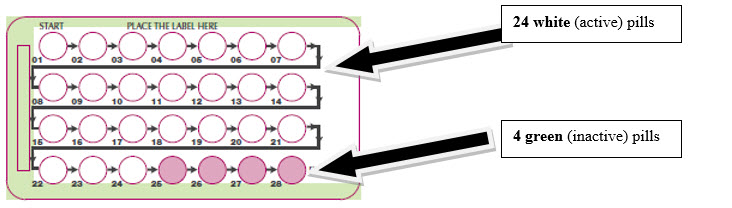
- 24 white (active) pills with hormone for Week 1 through Week 3 and the first 3 days of Week 4 (Days 1-24)
- 4 green (inactive) pills without hormones for the last 4 days of Week 4 (Days 25-28).
Step 2.
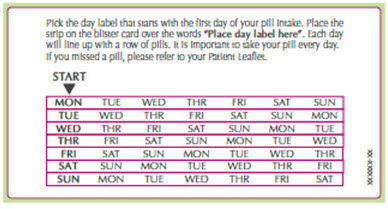
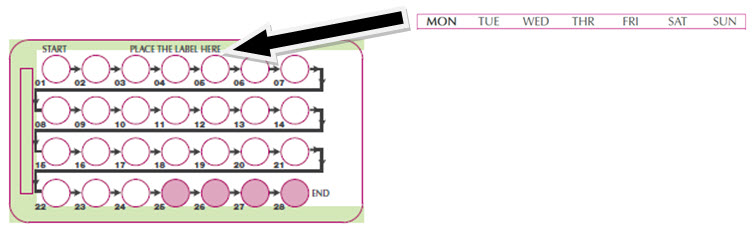
Place the day label strip (see Figure B) that starts with the first day of your period (Day 1) on top of the pill blister pack over "Place the label here". See Figure C. For example, if your period begins on Monday, place the day label strip with Monday as the first day on the top of your pill pack. See Figure C.
Step 3.
Remove the white pill by pressing the pill through the foil in the bottom of the pill blister pack. Continue taking the white pills for 24 days.
Step 4.
In the middle of Week 4 start taking the green pills. Take the green pill for 4 days. Your period should start during this time.
Step 5.
When you have taken all of the green pills in your pill pack, get a new pill pack and start taking the white pills from the new pill blister pack at your usual time the following day, starting with the Day 1 pill.
For a Day 1 start:
- Begin your next pill pack on the same day of the week as your first cycle pill pack.
What should I do if I miss any SLYND pills?
If you miss 1 white pill (active pills):
- Take it as soon as you remember. Take the next pill at your regular time. This means you may take 2 pills in 1 day.
- Then continue taking 1 pill every day until you finish the pack.
- You do not need to use a back-up birth control method if you have sex.
If you miss 2 or more white pills (active pills), follow these steps:
- Take a pill as soon as you remember. Take the next pill at your regular time. This means you may take 2 pills in 1 day.
- Then continue to take 1 pill every day until you finish the pack (this will mean one or more missed white pills will remain in the blister pack).
- Use a non-hormonal birth control method (such as a condom or spermicide) as a back-up if you have sex during the first 7 days after missing your pills.
If you miss 1 or more green pills (inactive pill):
- You do not need to take 1 or more missed green pills. Take the next green pill at your regular time, every day until you finish the pack (this means 1 or more missed green pill will remain in the blister pack).
If you have any questions or are unsure about the information in this leaflet, call your healthcare provider. You can ask your healthcare provider or pharmacist for information about SLYND that is written for health professionals.
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for: Exeltis USA, Inc. Florham Park, NJ 07932
Manufactured by: Laboratorios León Farma, S. A., Navatejera, Spain 24008
Issued: 05/2019
-
PRINCIPAL DISPLAY PANEL - 4 mg Tablet Blister Card Carton
NDC: 0642-7470-06
Rx only
Slynd®
(drospirenone) tablets, 4 mgExeltis
6 blister cards with 28 tables per blister card / Oral Use
KEEP OUT OF THE SIGHT AND REACH OF CHILDREN
-
INGREDIENTS AND APPEARANCE
SLYND
drospirenone tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0642-7470 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Drospirenone (UNII: N295J34A25) (Drospirenone - UNII:N295J34A25) Drospirenone 4 mg Inactive Ingredients Ingredient Name Strength Anhydrous Lactose (UNII: 3SY5LH9PMK) 17.5 mg Microcrystalline cellulose (UNII: OP1R32D61U) 33.02 mg Silicon Dioxide (UNII: ETJ7Z6XBU4) 0.29 mg Magnesium Stearate (UNII: 70097M6I30) 0.29 mg Polyvinyl Alcohol, Unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Product Characteristics Color WHITE Score no score Shape ROUND Size 5mm Flavor Imprint Code D;E Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0642-7470-02 1 in 1 CARTON 09/01/2019 1 NDC: 0642-7470-01 28 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 0642-7470-03 3 in 1 CARTON 09/01/2019 2 NDC: 0642-7470-01 28 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 0642-7470-06 6 in 1 CARTON 09/01/2019 3 NDC: 0642-7470-01 28 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211367 06/06/2019 Labeler - Exeltis USA, Inc. (071170534) Establishment Name Address ID/FEI Business Operations Laboratorios Leon Farma, S.A. 467782459 MANUFACTURE(0642-7470) , ANALYSIS(0642-7470)
Trademark Results [SLYND]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SLYND 88802490 not registered Live/Pending |
Chemo Research, S.L. 2020-02-19 |
 SLYND 88274384 not registered Live/Pending |
CHEMO RESEARCH, S.L. 2019-01-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.