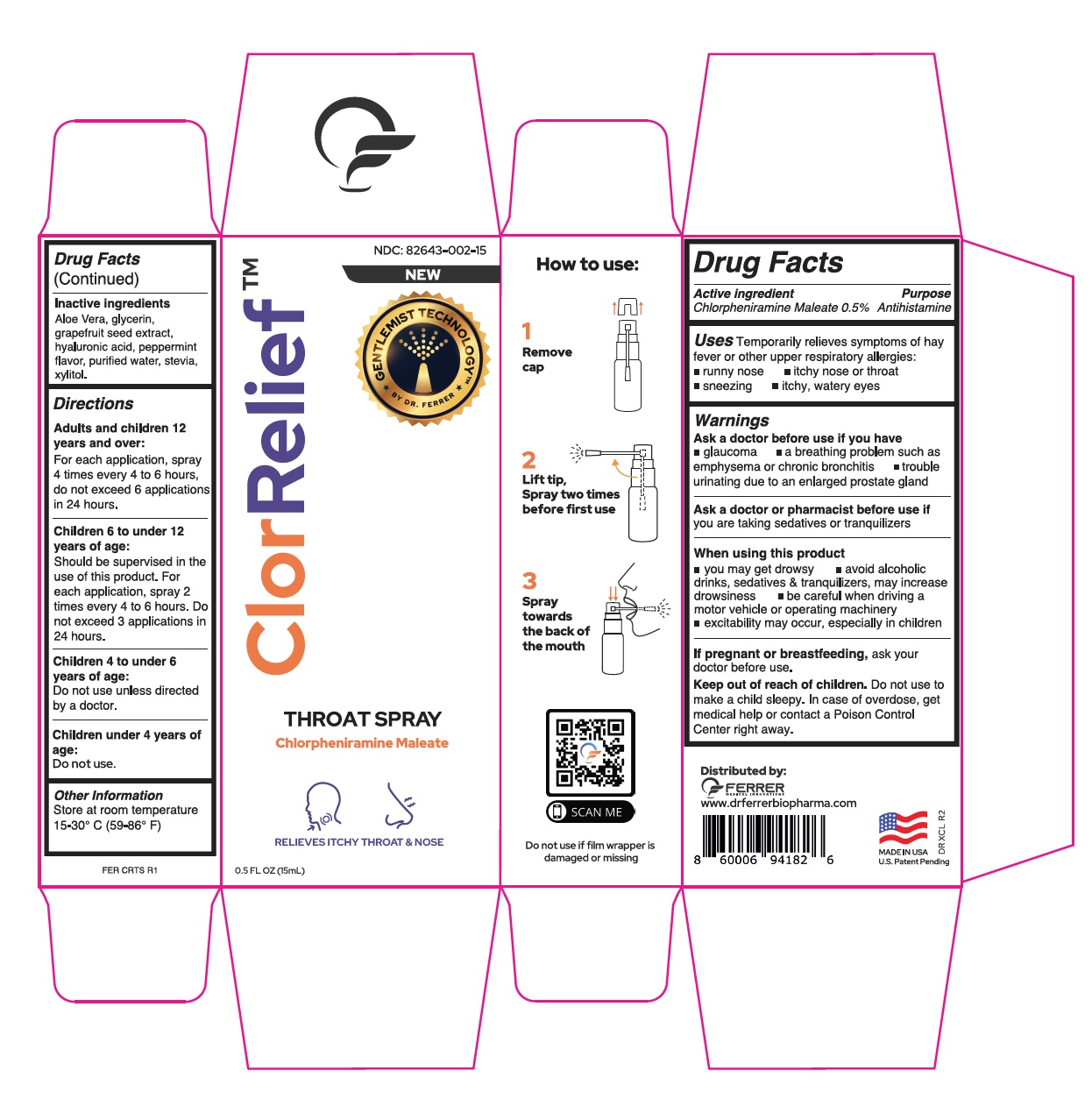
CLORRELIEF- chlorpheniramine maleate aerosol, spray
ClorRelief by
Drug Labeling and Warnings
ClorRelief by is a Otc medication manufactured, distributed, or labeled by FERRER MEDICAL INNOVATIONS LLC, Xlear Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Uses
-
Warnings
Ask a doctor before use if you have
glaucoma a breathing problem such as emphysema or chronic bronchitis trouble urinating due to an enlarged prostate gland
- Inactive ingredients
-
Directions
Adults and children 12 years and over:
For each application, spray 4 times every 4 to 6 hours,
do not exceed 6 applications in 24 hours.
Children 6 to under 12 years of age:
Should be supervised in the use of this product. For each application, spray 2 times every 4 to 6 hours. Do not exceed 3 applications in 24 hours.Children 4 to under 6 years of age:
Do not use unless directed by a doctor.Children under 4 years of age:
Do not use. - Other Information
- ClorRelief Box
-
INGREDIENTS AND APPEARANCE
CLORRELIEF
chlorpheniramine maleate aerosol, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82643-002 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA WHOLE (UNII: KIZ4X2EHYX) PEPPERMINT (UNII: V95R5KMY2B) GRAPEFRUIT SEED OIL (UNII: 598D944HOL) HYALURONIC ACID (UNII: S270N0TRQY) STEVIA LEAF (UNII: 6TC6NN0876) Product Characteristics Color Score Shape Size Flavor SPEARMINT, PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82643-002-15 1 in 1 CARTON 04/24/2023 1 15 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 04/21/2023 Labeler - FERRER MEDICAL INNOVATIONS LLC (041608434) Registrant - FERRER MEDICAL INNOVATIONS LLC (041608434) Establishment Name Address ID/FEI Business Operations Xlear Inc. 839884058 manufacture(82643-002)
Trademark Results [ClorRelief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CLORRELIEF 97228913 not registered Live/Pending |
FERRER MEDICAL INNNOVATIONS,LLC 2022-01-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.