BONJESTA- doxylamine succinate and pyridoxine hydrochloride tablet, extended release
BONJESTA by
Drug Labeling and Warnings
BONJESTA by is a Prescription medication manufactured, distributed, or labeled by Duchesnay USA, Inc., Duchesnay Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BONJESTA safely and effectively. See full prescribing information for BONJESTA.
BONJESTA (doxylamine succinate and pyridoxine hydrochloride), extended-release tablets, for oral use.
Initial U.S. Approval: 1976RECENT MAJOR CHANGES
Warnings and Precautions, Interference with Urine Screen for Methadone, Opiates and Phencyclidine Phosphate (PCP) (5.3) 06/2018
INDICATIONS AND USAGE
BONJESTA is a fixed dose combination drug product of 20 mg doxylamine succinate, an antihistamine, and 20 mg pyridoxine hydrochloride, a Vitamin B6 analog, indicated for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management. (1)
DOSAGE AND ADMINISTRATION
On Day 1, take one tablet at bedtime. On Day 2, if symptoms are not adequately controlled, the dose can be increased to one tablet in the morning and one tablet at bedtime. The maximum recommended dose is two tablets daily, one in the morning and one at bedtime, as described in the full prescribing information. (2)
DOSAGE FORMS AND STRENGTHS
Extended-release tablets containing 20 mg doxylamine succinate and 20 mg pyridoxine hydrochloride. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Somnolence: BONJESTA may cause somnolence. Avoid engaging in activities requiring complete mental alertness, such as driving or operating heavy machinery, while using BONJESTA until cleared to do so by a healthcare provider (5.1)
- Central nervous system (CNS) depressants: Concurrent use with alcohol or other CNS depressants is not recommended (5.1)
- Anticholinergic actions: Use with caution in patients with asthma, increased intraocular pressure, narrow angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction and urinary bladder-neck obstruction (5.2)
- Interference with urine drug screen: BONJESTA may interfere with urine screening for methadone, opiates and PCP (5.3)
ADVERSE REACTIONS
The most common adverse reaction (≥5 percent and exceeding the rate in placebo) with combination 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride tablets is somnolence. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Duchesnay Inc. at 1-855-722-7734 or medicalinfo@duchesnayusa.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Severe drowsiness can occur when used in combination with alcohol or other sedating medications. (7)
USE IN SPECIFIC POPULATIONS
BONJESTA is intended for use in pregnant women. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence and Severe Drowsiness
5.2 Concomitant Medical Conditions
5.3 Interference with Urine Screen for Methadone, Opiates and Phencyclidine Phosphate (PCP)
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drug Interactions
7.2 Drug-Food Interactions
7.3 False Positive Urine Tests for Methadone, Opiates and PCP
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdose
10.2 Management of Overdose
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
Somnolence
Interference with urine drug screening
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Information
Initially, take one BONJESTA extended-release tablet orally at bedtime (Day 1). If this dose adequately controls symptoms the next day, continue taking one tablet daily at bedtime only. However, if symptoms persist on Day 2, increase the daily dose to one tablet in the morning and one tablet at bedtime. The maximum recommended dose is two tablets per day, one in the morning and one at bedtime.
Take on an empty stomach with a glass of water [see Clinical Pharmacology (12.3)]. Swallow tablets whole. Do not crush, chew, or split BONJESTA tablets.
Take daily and not on an as needed basis. Reassess the woman for continued need for BONJESTA as her pregnancy progresses.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
BONJESTA is contraindicated in women with any of the following conditions:
- Known hypersensitivity to doxylamine succinate, other ethanolamine derivative antihistamines, pyridoxine hydrochloride or any inactive ingredient in the formulation
- Monoamine oxidase (MAO) inhibitors intensify and prolong the adverse central nervous system effects of BONJESTA [see Drug Interactions (7.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Somnolence and Severe Drowsiness
BONJESTA may cause somnolence due to the anticholinergic properties of doxylamine succinate, an antihistamine. Women should avoid engaging in activities requiring complete mental alertness, such as driving or operating heavy machinery, while using BONJESTA until cleared to do so by their healthcare provider.
BONJESTA use is not recommended if a woman is concurrently using central nervous system (CNS) depressants including alcohol. The combination may result in severe drowsiness leading to falls or accidents [see Drug Interactions (7.1)].
5.2 Concomitant Medical Conditions
BONJESTA has anticholinergic properties and, therefore, should be used with caution in women with: asthma, increased intraocular pressure, narrow angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction or urinary bladder-neck obstruction.
5.3 Interference with Urine Screen for Methadone, Opiates and Phencyclidine Phosphate (PCP)
There have been reports of false positive urine screening tests for methadone, opiates, and PCP with doxylamine succinate/pyridoxine hydrochloride use [see Drug Interactions (7.3)].
-
6 ADVERSE REACTIONS
-
The following adverse reactions are discussed elsewhere in the labeling:
- Somnolence [see Warnings and Precautions (5.1)]
- Falls or other accidents resulting from the effect of the combined use of BONJESTA with CNS depressants including alcohol [see Warnings and Precautions (5.1)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety and efficacy of combination 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride tablets compared to placebo was studied in a double-blind, randomized, multi-center trial in 261 women with nausea and vomiting of pregnancy. The mean gestational age at enrollment was 9.3 weeks, range 7 to 14 weeks gestation [see Clinical Studies (14)]. Adverse reactions that occurred at an incidence ≥5 percent and exceeded the incidence for placebo are summarized in Table 1.
Table 1: Number (Percent) of Women with ≥ 5 Percent Adverse Reactions in a 15-Day Placebo-Controlled Trial of Combination 10 mg Doxylamine Succinate and 10 mg Pyridoxine Hydrochloride Tablets (Only Those Adverse Reactions Occurring at an Incidence ≥ 5 Percent and at a Higher Incidence than Placebo are Shown)
Adverse Reaction
Combination 10 mg Doxylamine Succinate and 10 mg Pyridoxine Hydrochloride Tablets
(N = 133)
Placebo
(n = 128)
Somnolence
19 (14.3%)
15 (11.7%)
6.2 Postmarketing Experience
The following adverse events, listed alphabetically, have been identified during post-approval use of the combination of 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorders: dyspnea, palpitation, tachycardia
Ear and labyrinth disorders: vertigo
Eye disorders: vision blurred, visual disturbances
Gastrointestinal disorders: abdominal distension, abdominal pain, constipation, diarrhea
General disorders and administration site conditions: chest discomfort, fatigue, irritability, malaise
Immune system disorders: hypersensitivity
Nervous system disorders: dizziness, headache, migraines, paresthesia, psychomotor hyperactivity
Psychiatric disorders: anxiety, disorientation, insomnia, nightmares
Renal and urinary disorders: dysuria, urinary retention
Skin and subcutaneous tissue disorders: hyperhidrosis, pruritus, rash, rash maculopapular
-
7 DRUG INTERACTIONS
7.1 Drug Interactions
Use of BONJESTA is contraindicated in women who are taking monoamine oxidase inhibitors (MAOIs), which prolong and intensify the adverse central nervous system effects (the anticholinergic effects) of antihistamines. Concurrent use of alcohol and other CNS depressants (such as hypnotic sedatives and tranquilizers) with BONJESTA is not recommended.
7.2 Drug-Food Interactions
A food-effect trial demonstrated that the delay in the onset of action of BONJESTA may be further delayed, and a reduction in absorption may occur when tablets are taken with food [see Dosage and Administration (2), Clinical Pharmacology (12.3)]. Therefore, BONJESTA should be taken on an empty stomach with a glass of water [see Dosage and Administration (2)].
7.3 False Positive Urine Tests for Methadone, Opiates and PCP
False positive drug screens for methadone, opiates, and PCP can occur with doxylamine succinate/pyridoxine hydrochloride use. Confirmatory tests, such as Gas Chromatography Mass Spectrometry (GC-MS), should be used to confirm the identity of the substance in the event of a positive immunoassay result.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
BONJESTA is intended for the treatment of nausea and vomiting of pregnancy in women who do not respond to conservative management. Maternal risks are discussed throughout the labeling. No increased risk for congenital malformations has been reported in epidemiologic studies in pregnant women.
In the U.S. general population, the estimated background risks for major birth defects and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.
Data
Human Data
The combination of doxylamine succinate and pyridoxine hydrochloride has been the subject of many epidemiological studies (cohort, case control and meta-analyses) designed to detect possible teratogenicity. A meta-analysis of 16 cohort and 11 case-control studies published between 1963 and 1991 reported no increased risk for malformations from first trimester exposures to doxylamine succinate and pyridoxine hydrochloride, with or without dicyclomine hydrochloride. A second meta-analysis of 12 cohort and 5 case-control studies published between 1963 and 1985 reported no statistically significant relationships between fetal abnormalities and the first trimester use of the combination of doxylamine succinate and pyridoxine hydrochloride with or without dicyclomine hydrochloride.
8.2 Lactation
Risk Summary
Women should not breastfeed while using BONJESTA.
The molecular weight of doxylamine succinate is low enough that passage into breast milk can be expected. Excitement, irritability and sedation have been reported in nursing infants presumably exposed to doxylamine succinate through breast milk. Infants with apnea or other respiratory syndromes may be particularly vulnerable to the sedative effects of BONJESTA resulting in worsening of their apnea or respiratory conditions.
Pyridoxine hydrochloride is excreted into breast milk. There have been no reports of adverse events in infants presumably exposed to pyridoxine hydrochloride through breast milk.
8.4 Pediatric Use
The safety and effectiveness of BONJESTA in children under 18 years of age have not been established.
Fatalities have been reported from doxylamine overdose in children. The overdose cases have been characterized by coma, grand mal seizures and cardiorespiratory arrest. Children appear to be at a high risk for cardiorespiratory arrest. A toxic dose for children of more than 1.8 mg/kg has been reported. A 3 year old child died 18 hours after ingesting 1,000 mg doxylamine succinate. However, there is no correlation between the amount of doxylamine ingested, the doxylamine plasma level and clinical symptomatology.
-
10 OVERDOSAGE
10.1 Signs and Symptoms of Overdose
BONJESTA is an extended-release formulation, therefore, signs and symptoms of intoxication may not be apparent immediately.
Signs and symptoms of overdose may include restlessness, dryness of mouth, dilated pupils, sleepiness, vertigo, mental confusion and tachycardia.
At toxic doses, doxylamine exhibits anticholinergic effects, including seizures, rhabdomyolysis, acute renal failure and death.
-
11 DESCRIPTION
BONJESTA extended-release tablets consist of an enteric-coated core containing 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride, and an immediate release coating of 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride.
BONJESTA tablets are round, pink, film-coated, multilayer, extended-release tablets containing a total of 20 mg doxylamine succinate and 20 mg pyridoxine hydrochloride. Tablets are imprinted on one side with the pink image of a pregnant woman and a “D” on the other side.
Inactive ingredients are as follows: ammonium hydroxide, n-butanol, carnauba wax powder, colloidal silicon dioxide, croscarmellose sodium, D&C Red#27 aluminum lake, denatured alcohol, ferrosoferric oxide, FD&C Blue #2 aluminum lake, hypromellose, iron oxide red, isopropyl alcohol, magnesium stearate, magnesium trisilicate, methacrylic acid copolymer, microcrystalline cellulose 102, PEG 3350, propylene glycol, shellac glaze, simethicone, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate.
BONJESTA is certified Kosher, Kosher for Passover
 and Halal
and Halal  .
.Doxylamine Succinate
Doxylamine succinate is classified as an antihistamine. The chemical name for doxylamine succinate is ethanamine, N,N-dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]-, butanedioate (1:1). The empirical formula is C17H22N2O C4H6O4 and the molecular mass is 388.46. The structural formula is:
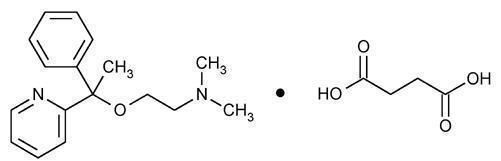
Doxylamine succinate is a white to creamy white powder that is very soluble in water and alcohol, freely soluble in chloroform and very slightly soluble in ether and benzene.
Pyridoxine Hydrochloride
Pyridoxine hydrochloride is a vitamin B6 analog. The chemical name for pyridoxine hydrochloride is 3,4-pyridinedimethanol, 5-hydroxy-6-methyl-, hydrochloride. The empirical formula is C8H11NO3 HCl and the molecular mass is 205.64. The structural formula is:
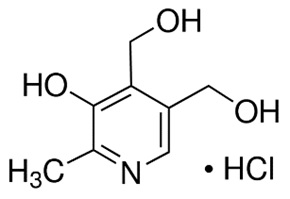
Pyridoxine hydrochloride is a white or practically white crystalline powder that is freely soluble in water, slightly soluble in alcohol and insoluble in ether.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
The pharmacokinetics of BONJESTA has been characterized in healthy non-pregnant adult women.
Absorption
In a single-dose, crossover clinical trial conducted in 48 healthy, premenopausal women under fasting conditions, one BONJESTA (20 mg doxylamine succinate and 20 mg pyridoxine) tablet was bioequivalent to two combination tablets of 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride based on the exposure (AUC) and peak concentration (Cmax) of doxylamine and baseline corrected pyridoxal 5′-phosphate. Mean ± SD plasma (whole blood for pyridoxal) pharmacokinetic (PK) parameters are summarized in Table 2.
Table 2 – Mean ± SD Single-Dose Pharmacokinetics of BONJESTA in Healthy Premenopausal Adult Women - * Median (range)
- † Baseline corrected values
- ‡ N=46 for AUC0-inf
BONJESTA
Mean±SDAUC0-t
(ngh/mL)AUC0-inf
(ngh/mL)AUC0-72
(ngh/mL)Cmax
(ng/mL)Tmax*
(h)Doxylamine
N=48 1367.0 ±
356.71425.8 ±
405.1--- 92.3 ±
15.74.5
(2.5-5.5)Pyridoxine
N=47 42.3 ±
14.742.5 ±
14.7--- 47.1 ±
18.70.5
(0.5-4.7)Pyridoxal†
N=48‡ 203.7 ±
51.7233.6 ±
55.9--- 58.9 ±
17.03.0
(0.8-5.0)Pyridoxal 5′-Phosphate†
N=48 --- --- 1076.2 ±
382.230.1 ±
9.29.0
(3.0-16.0)In a multiple-dose, crossover clinical trial conducted in 31 healthy, premenopausal women, one BONJESTA (20 mg doxylamine succinate and 20 mg pyridoxine) tablet given twice daily for 11 days was bioequivalent to one combination tablet of 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride given three times daily (1 tablet in the morning, 1 tablet in the afternoon and 2 tablets at bedtime), based on the exposure (AUC) and peak concentration (Cmax) of doxylamine and baseline corrected pyridoxal 5′-phosphate. Mean ± SD plasma (whole blood for pyridoxal) PK parameters are summarized in Table 3.
Table 3 – Mean ± SD Multiple-Dose (Day 11) Pharmacokinetic Parameters of BONJESTA (given twice daily) in Healthy Premenopausal Adult Women - * Median (range)
- † Baseline corrected values
BONJESTA
Mean±SDAUC0-24
(ngh/mL)AUC0-12
(ngh/mL)AUC0-6
(ngh/mL)Cmax
(ng/mL)Tmax*
(h)Doxylamine
N=34 2879.4 ±
696.01573.2 ±
406.5883.6 ±
228.5173.6 ±
45.53.5
(1.0-20.0)Pyridoxine
N=34 80.0 ±
22.746.3 ±
15.445.3 ±
16.348.2 ±
23.71.5
(0.3-16.5)Pyridoxal†
N=34 1511.3 ±
300.0848.1 ±
183.6647.2 ±
149.6189.6 ±
48.33.0
(2.0-15.0)Pyridoxal 5′-Phosphate†
N=34 1742.3 ±
554.3831.7 ±
274.5426.2 ±
144.085.9 ±
26.215.0
(2.0-24.0)Food Effect
In a single-dose, crossover clinical trial conducted in 23 healthy, premenopausal women, the administration of a high fat, high calorie meal delayed the absorption of doxylamine, pyridoxine, and pyridoxine metabolites. This delay is associated with lower peak concentrations of doxylamine, pyridoxine, and pyridoxal. The extent of absorption for pyridoxine was decreased.
The effect of food on the peak concentration and the extent of absorption of the pyridoxine component is more complex because the pyridoxine metabolites such as pyridoxal, pyridoxamine, pyridoxal 5′-phosphate, and pyridoxamine 5′-phosphate also contribute to the biological activity. Food significantly reduces the bioavailability of pyridoxine, lowering its Cmax and AUC by approximately 67% and 37%, respectively, compared to fasting conditions. Similarly, food significantly reduces pyridoxal Cmax by approximately 46% compared to fasting conditions. In contrast, food did not affect pyridoxal 5′-phosphate Cmax and AUC.
Table 4 – Mean ± SD Pharmacokinetic Parameters of Doxylamine and Pyridoxine Metabolites Following a Single Dose Administration of BONJESTA Under Fed and Fasted Conditions in Healthy Premenopausal Adult Women - * Profile of Subject 20 was excluded
- † Median (range)
- ‡ Baseline corrected values
BONJESTA
N=23AUC0-t (ngh/mL) AUC0-inf (ngh/mL) Cmax
(ng/mL)Tmax*,†
(h)T1/2el
(h)Doxylamine
Mean±SDFasted 1273.7 ±
276.21321.9 ±
315.585.9 ±
10.63.5
(2.5-5.5)11.9 ±
2.2Fed 1242.8 ±
254.01281.4 ±
282.964.5 ±
15.26.5
(2.0-24.0)12.7 ±
2.60Pyridoxine
Mean±SDFasted 34.7 ±
10.635.1 ±
8.538.9 ±
19.30.8
(0.3-4.3)0.4 ±
0.2Fed 22.8 ±
9.927.0 ±
10.112.7 ±
5.78.0
(1.0-21.0)1.2 ±
2.4Pyridoxal‡
Mean±SDFasted 209.4 ±
30.0244.0 ±
32.562.0 ±
17.82.3
(0.8-5.0)8.0 ±
1.7Fed 204.2 ±
25.7249.2 ±
43.033.1 ±
6.16.0
(1.0-21.0)12.5 ±
7.6Pyridoxal 5′-phosphate‡
Mean±SDFasted 1021.7 ±
318.5--- 27.4 ±
7.75.0
(3.0-71.8)--- Fed 1064.6 ±
386.9--- 30.2 ±
10.016.0
(6.0-22.0)--- Distribution
Pyridoxine is highly protein bound, primarily to albumin. Its main active metabolite, pyridoxal 5′-phosphate (PLP) accounts for at least 60% of circulating vitamin B6 concentrations.
Metabolism
Doxylamine is biotransformed in the liver by N-dealkylation to its principle metabolites N-desmethyl-doxylamine and N, N-didesmethyldoxylamine.
Pyridoxine is a prodrug primarily metabolized in the liver.
Excretion
The principle metabolites of doxylamine, N-desmethyl-doxylamine and N, N-didesmethyldoxylamine, are excreted by the kidney.
The terminal elimination half-life of doxylamine and pyridoxine are 11.9 hours and 0.4 hours, respectively (see Table 5).
Table 5 – Terminal Elimination Half-Life (T1/2el) for BONJESTA Administered as a Single Dose under Fasting Conditions in Healthy Premenopausal Adult Women (N=23) - * N=12
- † Baseline corrected value
BONJESTA
T1/2el (h)Doxylamine 11.9 ± 2.2 Pyridoxine 0.4 ± 0.2* Pyridoxal 8.0 ± 1.7† Use in Specific Populations
Race: No pharmacokinetic studies have been conducted related to race.
Hepatic Impairment: No pharmacokinetic studies have been conducted in hepatic impaired patients.
Renal Impairment: No pharmacokinetic studies have been conducted in renal impaired patients.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
Carcinogenicity
Two-year carcinogenicity studies in rats and mice have been conducted with doxylamine succinate. Doxylamine succinate is not likely to have human carcinogenic potential. The carcinogenic potential of pyridoxine hydrochloride has not been evaluated.
-
14 CLINICAL STUDIES
There have been no efficacy and safety trials conducted with BONJESTA.
A double-blind, randomized, multi-center, placebo-controlled study was conducted to support the safety and efficacy of 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride tablets (a different formulation and dosage strength than BONJESTA) in the treatment of nausea and vomiting of pregnancy. Adult women 18 years of age or older and 7 to 14 weeks gestation (median 9 weeks of gestation) with nausea and vomiting of pregnancy were randomized to 14 days of 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride tablets or placebo. Two tablets of 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride were administered at bedtime on Day 1. If symptoms of nausea and vomiting persisted into the afternoon hours of Day 2, the woman was directed to take her usual dose of two tablets at bedtime that night and, beginning on Day 3, to take one tablet in the morning and two tablets at bedtime. Based upon assessment of remaining symptoms at her clinic visit on Day 4 (± 1 day), the woman may have been directed to take an additional tablet mid-afternoon. A maximum of four tablets (one in the morning, one in the mid-afternoon and two at bedtime) were taken daily.
Over the treatment period, 19% of 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride tablet-treated women remained on 2 tablets daily, 21% received 3 tablets daily, and 60% received 4 tablets daily.
The primary efficacy endpoint was the change from baseline at Day 15 in the Pregnancy Unique-Quantification of Emesis (PUQE) score. The PUQE score incorporates the number of daily vomiting episodes, number of daily heaves, and length of daily nausea in hours, for an overall score of symptoms rated from 3 (no symptoms) to 15 (most severe).
At baseline, the mean PUQE score was 9.0 in the 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride tablets arm and 8.8 in the placebo arm. There was a 0.7 (95% confidence interval 0.2 to 1.2 with p-value 0.006) mean decrease (improvement in nausea and vomiting symptoms) from baseline in PUQE score at Day 15 with 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride tablets compared to placebo (see Table 6).
Table 6 – Change from Baseline in the Primary Endpoint, Pregnancy Unique-Quantification of Emesis (PUQE) Score at Day 15. (Intent-to-Treat Population with Last-Observation Carried Forward) - * The Pregnancy-Unique Quantification of Emesis and Nausea (PUQE) score incorporated the number of daily vomiting episodes, number of daily heaves, and length of daily nausea in hours, for an overall score of symptoms rated from 3 (no symptoms) to 15 (most severe). Baseline was defined as the PUQE score completed at the enrollment visit.
PUQE Score* Combination 10 mg Doxylamine Succinate and 10 mg Pyridoxine Hydrochloride Tablets
N=131
Placebo
N=125
Treatment Difference
[95% Confidence Interval]Baseline
Change from baseline at Day 159.0 ± 2.1
-4.8 ± 2.78.8 ± 2.1
-3.9 ± 2.6-0.7 [-1.2, -0.2] -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How supplied
BONJESTA extended-release tablets are supplied in a high-density polyethylene bottle with a polypropylene child-resistant cap and a silica gel desiccant canister. Each pink, round, film-coated, extended-release tablet contains 20 mg doxylamine succinate and 20 mg pyridoxine hydrochloride, and is imprinted on one side with the pink image of a pregnant woman and a "D" on the other side. BONJESTA tablets are provided as follows:
NDC: 55494-120-60 Bottles of 60
NDC: 55494-120-10 Bottles of 100 -
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information)
Somnolence
Inform women to avoid engaging in activities requiring complete mental alertness, such as driving or operating heavy machinery, while using BONJESTA until cleared to do so.
Inform women of the importance of not taking BONJESTA with alcohol or sedating medications, including other antihistamines (present in some cough and cold medications), opiates and sleep aids because somnolence could worsen leading to falls or other accidents.
Interference with urine drug screening
Inform women that use of BONJESTA may result in false positive urine drug screening for methadone, opiates and PCP.
BONJESTA is a registered trademark of Duchesnay Inc.
U.S. Patent Nos. 9,089,489, 7,560,122, 9,375,404 & 9,526,703.
Manufactured by:
Duchesnay Inc.
950 boul. Michèle-Bohec
Blainville, Québec
Canada J7C 5E2Distributed by:
Duchesnay USA, Inc.
Bryn Mawr, PA, 19010
Tel: 1-855-722-7734
Fax: 1-888-588-8508
www.duchesnayusa.com©2018, Duchesnay Inc. All rights reserved.
-
Patient Package Insert
Patient Information
BONJESTA (bonn jest ah)
(doxylamine succinate and pyridoxine hydrochloride)
extended-release tablets, for oral use
What is BONJESTA?
- BONJESTA is a prescription medicine used to treat nausea and vomiting of pregnancy in women who have not improved with change in diet or other non-medicine treatments.
- It is not known if BONJESTA is safe and effective in women with severe nausea and vomiting of pregnancy, a condition called hyperemesis gravidarum. Women with this condition may need to be hospitalized.
- It is not known if BONJESTA is safe and effective in children under 18 years of age.
Do not take BONJESTA if you:
- are allergic to doxylamine succinate, other ethanolamine derivative antihistamines, pyridoxine hydrochloride or any of the ingredients in BONJESTA. See the end of this Patient Information leaflet for a complete list of ingredients in BONJESTA.
- take monoamine oxidase inhibitors (MAOIs). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including Marplan, Nardil, Emsam, Eldepryl, Zelapar, and Parnate.
Before taking BONJESTA, tell your healthcare provider about all of your medical conditions, including if you:
- have asthma.
- have eye problems called increased intraocular pressure or narrow angle glaucoma.
- have a stomach problem called stenosing peptic ulcer or pyloroduodenal obstruction.
- have a bladder problem called urinary bladder-neck obstruction.
- are breastfeeding or plan to breastfeed. BONJESTA can pass into your breast milk and may harm your baby. You should not breastfeed while using BONJESTA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take BONJESTA?
- Talk to your healthcare provider about how much BONJESTA to take and when to take it.
- Take BONJESTA everyday as prescribed by your healthcare provider. Do not stop taking BONJESTA without talking to your healthcare provider first.
-
See the following schedule for the right way you should start taking BONJESTA:
- Start with 1 tablet by mouth at bedtime. If your nausea and vomiting is better or controlled on Day 2, continue to take 1 tablet each day at bedtime.
- If you still have nausea and vomiting on Day 2, start taking 1 tablet in the morning and 1 tablet at bedtime each day.
- Do not take more than 2 tablets (1 in the morning and 1 at bedtime) each day.
- Take BONJESTA on an empty stomach with a glass of water.
- Take BONJESTA tablets whole. Do not crush, chew, or break BONJESTA tablets before swallowing. If you cannot swallow BONJESTA tablets whole, tell your healthcare provider.
- If you take too much BONJESTA (overdose), you may have the following symptoms: restlessness, dry mouth, the pupils of your eyes become larger (dilated), sleepiness, dizziness, confusion, fast heart rate, seizures, muscle pain or weakness, urination changes and build-up of fluid in the body. If you have these symptoms and they are severe, they may lead to death. If you take too much BONJESTA, call your poison control center at 1-800-222-1222.
What are the possible side effects of BONJESTA?
BONJESTA may cause serious side effects, including drowsiness.
Drowsiness is a common side effect when taking BONJESTA, but can also be severe:
- Do not drive, operate heavy machinery, or do other activities that need your full attention unless your healthcare provider says that you may do so.
- Do not drink alcohol, or take other central nervous system depressants such as cough and cold medicines, certain pain medicines, and medicines that help you sleep while you take BONJESTA. Severe drowsiness can happen or become worse causing falls or accidents.
BONJESTA may cause false positive urine drug screening test for methadone, opiates and PCP.
These are not all the possible side effects of BONJESTA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store BONJESTA?
- Store BONJESTA at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep the bottle tightly closed to protect BONJESTA from moisture.
- The BONJESTA bottle contains a desiccant canister to help keep your medicine dry. Do not throw away the desiccant.
- Safely throw away medicine that is past the expiration date or no longer needed.
Keep BONJESTA and all medicines out of the reach of children.
General information about the safe and effective use of BONJESTA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about BONJESTA that is written for health professionals. Do not use BONJESTA for a condition for which it was not prescribed. Do not give BONJESTA to other people, even if they have the same symptoms that you have. It may harm them.
What are the ingredients in BONJESTA?
Active ingredient: doxylamine succinate (an antihistamine) and pyridoxine hydrochloride (vitamin B6).
Inactive ingredients: ammonium hydroxide, n-butanol, carnauba wax powder, colloidal silicon dioxide, croscarmellose sodium, D&C Red#27 aluminum lake, denatured alcohol, ferrosoferric oxide, FD&C Blue #2 aluminum lake, hypromellose, iron oxide red, isopropyl alcohol, magnesium stearate, magnesium trisilicate, methacrylic acid copolymer, microcrystalline cellulose 102, PEG 3350, propylene glycol, shellac glaze, simethicone, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate.
Distributed by: Duchesnay USA, Inc., Bryn Mawr, PA, 19010,
For more information, go to www.duchesnayusa.com or call 1-855-722-7734.This Patient Information has been approved by the U.S. Food and Drug Administration Issued: 06/2018
-
Package/Label Display Panel
Bottle Label-Outside Front Cover with Imprint Area for Lot & Expiry
NDC: 55494-120-60
Bonjesta®
(doxylamine succinate and pyridoxine hydrochloride)
Extended-release tablets 20mg/20mgWARNING: Swallow tablets whole.
Do not crush, chew, or split the tablets.60 extended-release tablets
DUCHESNAY
Rx only

Bottle Label – Inside Cover
-
INGREDIENTS AND APPEARANCE
BONJESTA
doxylamine succinate and pyridoxine hydrochloride tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55494-120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 20 mg Inactive Ingredients Ingredient Name Strength AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 27 (UNII: 2LRS185U6K) ALCOHOL (UNII: 3K9958V90M) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) ISOPROPYL ALCOHOL (UNII: ND2M416302) MAGNESIUM STEARATE (UNII: 70097M6I30) MAGNESIUM TRISILICATE (UNII: C2E1CI501T) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color pink Score no score Shape ROUND Size 8mm Flavor Imprint Code D Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55494-120-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/19/2018 2 NDC: 55494-120-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/19/2018 3 NDC: 55494-120-99 6 in 1 BOTTLE; Type 0: Not a Combination Product 02/19/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209661 02/19/2018 Labeler - Duchesnay USA, Inc. (032392244) Registrant - Duchesnay Inc. (240538306)
Trademark Results [BONJESTA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BONJESTA 87125569 5503748 Live/Registered |
Duchesnay Inc. 2016-08-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.