desoximetasone by Padagis Israel Pharmaceuticals Ltd DESOXIMETASONE spray
desoximetasone by
Drug Labeling and Warnings
desoximetasone by is a Prescription medication manufactured, distributed, or labeled by Padagis Israel Pharmaceuticals Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Desoximetasone Topical Spray, 0.25% safely and effectively. See full prescribing information for Desoximetasone Topical Spray, 0.25%.
Desoximetasone Topical Spray, 0.25%
Initial U.S. Approval: 1977INDICATIONS AND USAGE
Desoximetasone Topical Spray, 0.25% is a corticosteroid indicated for the treatment of plaque psoriasis in patients 18 years of age or older. (1)
DOSAGE AND ADMINISTRATION
- Apply a thin film to the affected skin areas twice daily. Rub in gently. (2)
- Desoximetasone Topical Spray, 0.25% should be discontinued when control is achieved. (2)
- Treatment beyond 4 weeks is not recommended. (2)
- Do not use if atrophy is present at the treatment site. (2)
- Do not use with occlusive dressings, unless directed by the physician. (2)
- Avoid use on the face, axilla or groin. (2)
- Desoximetasone Topical Spray, 0.25% is not for oral, ophthalmic, or intravaginal use. (2)
DOSAGE FORMS AND STRENGTHS
Spray, 0.25% w/w (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Desoximetasone Topical Spray, 0.25% can produce reversible HPA axis suppression with the potential for glucocorticosteroid insufficiency during or after treatment. (5.1)
- Cushing's syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can result from systemic absorption of topical corticosteroids. (5.1)
- Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. (5.1)
- Modify use if HPA axis suppression develops. (5.1)
- High potency corticosteroids, large treatment surface areas, prolonged use, use of occlusive dressings, altered skin barrier, liver failure and young age may predispose patients to HPA axis suppression. (5.1)
- Pediatric patients may be more susceptible to systemic toxicity when treated with topical corticosteroids. (5.1, 8.4)
- Desoximetasone Topical Spray, 0.25% is flammable; keep away from heat or flame. (5.5)
ADVERSE REACTIONS
The most common adverse reactions (≥ 1%) are application site dryness, application site irritation and application site pruritus. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Perrigo at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Effect on Endocrine System
5.2 Local Adverse Reactions with Topical Corticosteroids
5.3 Allergic Contact Dermatitis with Topical Corticosteroids
5.4 Concomitant Skin Infections
5.5 Flammable Contents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied/Storage
16.2 Instructions for the Pharmacist
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Apply Desoximetasone Topical Spray, 0.25% as a thin film to the affected skin areas twice daily. Rub in gently.
The treated skin area should not be bandaged or otherwise covered or wrapped unless directed by the physician.
Desoximetasone Topical Spray, 0.25% should be discontinued when control is achieved.
Treatment beyond 4 weeks is not recommended.
Do not use if atrophy is present at the treatment site.
Avoid use on the face, axilla or groin.
Desoximetasone Topical Spray, 0.25% is for external use only. It is not for oral, ophthalmic, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Effect on Endocrine System
Desoximetasone Topical Spray, 0.25% is a topical corticosteroid that has been shown to suppress the hypothalamic-pituitary-adrenal (HPA) axis.
Systemic absorption of topical corticosteroids can produce reversible HPA axis suppression with the potential for glucocorticosteroid insufficiency. This may occur during treatment or upon withdrawal of the topical corticosteroid.
In a study including 21 evaluable subjects 18 years of age or older with moderate to severe plaque psoriasis, adrenal suppression was identified in 1 out of 12 subjects having involvement of 10-15% of body surface area (BSA) and 2 out of 9 subjects having involvement of >15% of BSA after treatment with desoximetasone topical spray, 0.25% twice a day for 28 days. [see Clinical Pharmacology (12.2)]
Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. Factors that predispose a patient using a topical corticosteroid to HPA axis suppression include the use of high potency steroids, larger treatment surface areas, prolonged use, use of occlusive dressings, altered skin barrier, liver failure and young age.
An ACTH stimulation test may be helpful in evaluating patients for HPA axis suppression.
If HPA axis suppression is documented, an attempt should be made to gradually withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid. Manifestations of adrenal insufficiency may require supplemental systemic corticosteroids. Recovery of HPA axis function is generally prompt and complete upon discontinuation of topical corticosteroids.
Cushing’s syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can also result from systemic absorption of topical corticosteroids. Use of more than one corticosteroid-containing product at the same time may increase the total systemic corticosteroid exposure.
Pediatric patients may be more susceptible to systemic toxicity from use of topical corticosteroids. [see Use in Specific Populations (8.4)]
5.2 Local Adverse Reactions with Topical Corticosteroids
Local adverse reactions may be more likely to occur with occlusive use, prolonged use or use of higher potency corticosteroids. Reactions may include atrophy, striae, telangiectasias, burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria. Some local adverse reactions may be irreversible.
5.3 Allergic Contact Dermatitis with Topical Corticosteroids
Allergic contact dermatitis to any component of topical corticosteroids is usually diagnosed by a failure to heal rather than a clinical exacerbation. Clinical diagnosis of allergic contact dermatitis can be confirmed by patch testing.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In randomized, multicenter, prospective vehicle-controlled clinical trials, subjects with moderate to severe plaque psoriasis of the body applied desoximetasone topical spray, 0.25% or vehicle spray twice daily for 4 weeks. A total of 149 subjects applied desoximetasone topical spray, 0.25%.
Adverse reactions that occurred in ≥ 1% of subjects treated with desoximetasone topical spray, 0.25% were application site dryness (2.7%), application site irritation (2.7%) and application site pruritus (2.0%).
Another less common adverse reaction (<1% but >0.1%) was folliculitis.
Table 1. Number (%) of Subjects with Adverse Reactions Occurring in ≥ 1%
Desoximetasone Topical Spray, 0.25%
b.i.d.
(N = 149)
Vehicle spray
b.i.d.
(N = 135)
Number of Subjects with Adverse Reactions
13 (8.7%)
18 (13.3%)
Application site dryness
4 (2.7%)
7 (5.2%)
Application site irritation
4 (2.7%)
5 (3.7%)
Application site pruritus
3 (2.0%)
5 (3.7%)
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women.
Desoximetasone Topical Spray, 0.25% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels.
Desoximetasone has been shown to be teratogenic and embryotoxic in mice, rats, and rabbits when given by subcutaneous or dermal routes of administration at doses 3 to 30 times the human dose of desoximetasone topical spray, 0.25% based on a body surface area comparison.
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Because many drugs are excreted in human milk, caution should be exercised when Desoximetasone Topical Spray, 0.25% is administered to a nursing woman.
If used during lactation, Desoximetasone Topical Spray, 0.25% should not be applied on the chest to avoid accidental ingestion by the infant.
8.4 Pediatric Use
Safety and effectiveness of Desoximetasone Topical Spray, 0.25% in patients younger than 18 years of age have not been studied; therefore use in pediatric patients is not recommended. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing’s syndrome when they are treated with topical corticosteroids. They are therefore at greater risk of adrenal insufficiency during and/or after withdrawal of treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and children. [see Warnings and Precautions (5.1)]
HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema. [see Warnings and Precautions (5.1)]
8.5 Geriatric Use
Clinical studies of desoximetasone topical spray, 0.25% did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- 10 OVERDOSAGE
-
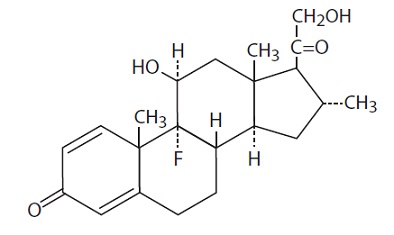
11 DESCRIPTION
Desoximetasone Topical Spray, 0.25% contains desoximetasone as the active ingredient.
Desoximetasone is a corticosteroid with the chemical name of pregna-1, 4-diene-3, 20-dione, 9-fluoro-11, 21-dihydroxy-16-methyl-, (11ß,16α)-.
Desoximetasone has the molecular formula of C22H29FO4 and a molecular weight of 376.47. The CAS Registry Number is 382-67-2.
The structural formula is:
Each gram of Desoximetasone Topical Spray, 0.25% contains 2.5 mg of desoximetasone in a clear, colorless liquid with the following inactive ingredients: glyceryl oleate, isopropyl alcohol (23.4%), isopropyl myristate, L-menthol, and mineral oil. Desoximetasone Topical Spray, 0.25% is co-packaged with a manual spray pump for installation by the pharmacist prior to dispensing to patients.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids play a role in cellular signaling, immune function, inflammation and protein regulation; however, the precise mechanism of action in psoriasis is unknown.
12.2 Pharmacodynamics
Vasoconstrictor studies performed with desoximetasone topical spray, 0.25% in healthy subjects indicate that it is in the high to super-high range of potency as compared with other topical corticosteroids.
The potential for hypothalamic-pituitary-adrenal (HPA) axis suppression was evaluated in a study of 24 adult subjects with moderate to severe plaque psoriasis. Desoximetasone topical spray, 0.25% was applied twice a day for 28 days. Twenty-one subjects had evaluable serum cortisol levels. The proportion of subjects demonstrating HPA axis suppression was 8.3% (1 out of 12) in subjects having psoriasis involvement of 10-15% of body surface area (BSA), and 22.2% (2 out of 9) in subjects having psoriasis involvement of > 15% of their BSA. In this study HPA axis suppression was defined as serum cortisol level ≤18 mcg/dL 30-min post cosyntropin stimulation. In the 2 subjects with available follow-up values, suppression reversed 28 days after the end of treatment.
12.3 Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
Plasma concentrations of desoximetasone were measured at two single random time points in the HPA axis suppression trial in 24 subjects with psoriasis [see Clinical Pharmacology (12.2)]. The mean (% Coefficient of Variation) concentration of desoximetasone was 449 pg/mL (86%) at Day 14 and 678 pg/mL (135%) at Day 28. The concentration time profile following application of desoximetasone topical spray, 0.25% is not known.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of desoximetasone topical spray, 0.25%.
In a 13-week repeat-dose toxicity study in rats, topical administration of desoximetasone spray at concentrations of 0.001, 0.005 and 0.02% BID (which corresponds to dose levels of 0.01, 0.05, or 0.2 mg/kg/dose BID, respectively) resulted in a toxicity profile consistent with long-term exposure to corticosteroids, including adrenal atrophy and histopathological changes in several organ systems indicative of severe immune suppression. A no observable adverse effect level (NOAEL) could not be determined in this study. Although the clinical relevance of the findings in animals to humans is not clear, sustained glucocorticoid-related immune suppression may increase the risk of infection and possibly the risk for carcinogenesis.
Desoximetasone revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames assay and Chinese hamster ovary cell chromosome aberration assay) and one in vivo genotoxicity test (mouse bone marrow micronucleus assay).
No evidence of impairment of male or female fertility was observed at subcutaneous desoximetasone doses up to 0.1 mg/kg/day (0.6 mg/m2/day) in Sprague-Dawley rats.
-
14 CLINICAL STUDIES
Two multi-center, randomized, double-blind, vehicle-controlled clinical trials were conducted in 239 subjects aged 18 years and older with moderate to severe plaque psoriasis of the body. In both trials, randomized subjects applied desoximetasone topical spray, 0.25% or vehicle spray to the affected areas twice daily for 4 weeks. Enrolled subjects had a minimum body surface area of involvement of 10%, and a Physician’s Global Assessment score (PGA) of 3 (moderate) or 4 (severe).
Efficacy was assessed at Week 4 as the proportion of subjects who were considered a Clinical Success (“clear” (0) or “almost clear” (1) according to the PGA scale). Table 2 presents the efficacy results.
Table 2. Number of Subjects (%) with Clinical Success (scored as clear or almost clear) at Week 4.
Parameter
Trial 1
Trial 2
Desoximetasone Topical Spray, 0.25%
N= 59
Vehicle
N= 60
Desoximetasone Topical Spray, 0.25%
N= 60
Vehicle
N= 60
Clinical Success
18 (30.5%)
3 (5.0%)
32 (53.3%)
11 (18.3%)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied/Storage
Desoximetasone Topical Spray, 0.25% is a clear colorless liquid supplied in white, opaque bottles with white, opaque screw caps in the following sizes:
- 100 mL (NDC 45802-807-98)
Store at controlled room temperature between 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Spray is flammable; avoid heat, flame or smoking when using this product.
Each unit is co-packaged with a manual spray pump for installation by the pharmacist.
16.2 Instructions for the Pharmacist
1. Remove the spray pump from the wrapper
2. Remove and discard the cap from the bottle
3. Keeping the bottle vertical, puncture seal, insert the spray pump into the bottle and turn clockwise until well-fastened
4. Dispense the bottle with the spray pump inserted
5. Label the bottle with “discard the product 30 days after dispensing”
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information and Instructions for Use) Inform patients of the following:
- Use this medication as directed by the physician.
- Desoximetasone Topical Spray, 0.25% is for external use only. Avoid use on the face, axilla or groin.
- Do not use this medication for any disorder other than that for which it was prescribed.
- Do not bandage or otherwise cover or wrap the treated skin so as to be occlusive.
- Report any signs of local or systemic adverse reactions to the physician.
- Do not use other corticosteroid-containing products with Desoximetasone Topical Spray, 0.25% without first consulting with the physician.
- Discontinue therapy when control is achieved. If no improvement is seen within 4 weeks, contact the physician.
- This medication is flammable; avoid heat, flame, or smoking when applying this product.
- Discard this product 30 days after dispensed by pharmacist.
Made in Israel
Manufactured By Perrigo
Yeruham, Israel
Distributed By
Perrigo®
Allegan, MI 49010 www.perrigo.com
Rev 08-18
: 7Y200 RC J1
-
Instructions for Use Desoximetasone Topical Spray, 0.25%
Important information:
Desoximetasone Topical Spray, 0.25% is for use on skin only.
Do not get Desoximetasone Topical Spray, 0.25% near or in your mouth, eyes or vagina.
Read the Instructions for Use that comes with Desoximetasone Topical Spray, 0.25% before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment.
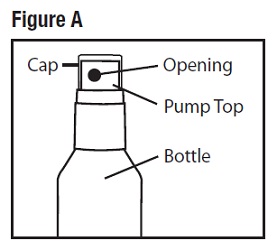
Parts of Desoximetasone Topical Spray, 0.25% bottle (See Figure A)
How to apply Desoximetasone Topical Spray, 0.25%:
Step 1: Remove the cap from the pump top.
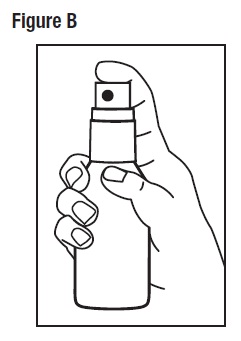
Step 2: Hold the bottle in an upright position while pointing the opening of the pump top in the direction of the affected area. To spray, push down on the pump top. Apply Desoximetasone Topical Spray, 0.25% to the affected area as instructed by your doctor. (See Figure B)
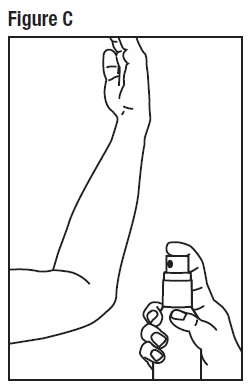
Step 3: Spray only enough Desoximetasone Topical Spray, 0.25% to cover the affected area, for example, the elbow. (See Figure C) Rub in Desoximetasone Topical Spray, 0.25% gently.
Repeat Steps 2 and 3 to apply Desoximetasone Topical Spray, 0.25% to other affected areas as instructed by your doctor.
Step 4: After applying Desoximetasone Topical Spray, 0.25%, place the cap back onto the pump top.
(See Figure D)
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
-
Patient Information
Desoximetasone Topical Spray, 0.25%
Important information: Desoximetasone Topical Spray, 0.25% is for use on skin only.
Do not get Desoximetasone Topical Spray, 0.25% near or in your eyes, mouth or vagina.
What is Desoximetasone Topical Spray, 0.25%?
- Desoximetasone Topical Spray, 0.25% is a prescription corticosteroid medicine used to treat plaque psoriasis of the body in people 18 years of age and older.
- You should not use Desoximetasone Topical Spray, 0.25% on your face, underarms (armpits), or groin areas.
- It is not known if Desoximetasone Topical Spray, 0.25% is safe and effective in children under 18 years of age. Desoximetasone Topical Spray, 0.25% should not be used in children under 18 years of age.
Before you use Desoximetasone Topical Spray, 0.25%, tell your doctor if you:
- are allergic to any of the ingredients in Desoximetasone Topical Spray, 0.25%. See the end of this leaflet for a list of the ingredients in Desoximetasone Topical Spray, 0.25%.
- have a skin infection. You may need medicine to treat the skin infection before you use Desoximetasone Topical Spray, 0.25%.
- have thinning of the skin (atrophy) at the treatment site
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if Desoximetasone Topical Spray, 0.25% will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Desoximetasone Topical Spray, 0.25% passes into your breast milk. Do not apply Desoximetasone Topical Spray, 0.25% to your chest area if you are breastfeeding. This will help to prevent your baby from accidentally getting Desoximetasone Topical Spray, 0.25% into the mouth.
Tell your doctor about all the medicines you take including prescription and over-the-counter medicines, vitamins and herbal supplements. Especially tell your doctor if you take other corticosteroid medicines by mouth or use other products on your skin that contain corticosteroids.
What should I avoid while using Desoximetasone Topical Spray, 0.25%?
Desoximetasone Topical Spray, 0.25% is flammable. Avoid heat, flames or smoking while applying Desoximetasone Topical Spray, 0.25% to your skin.
How should I use Desoximetasone Topical Spray, 0.25%?
- Use Desoximetasone Topical Spray, 0.25% exactly as your doctor tells you to use it.
- Apply Desoximetasone Topical Spray, 0.25% 2 times a day.
- Do not bandage, cover, or wrap the treated skin area.
- Desoximetasone Topical Spray, 0.25% should be used for the shortest amount of time needed to treat your plaque psoriasis. Tell your doctor if your skin condition is not getting better after 4 weeks of using Desoximetasone Topical Spray, 0.25%. You should not use Desoximetasone Topical Spray, 0.25% for longer than 4 weeks.
- Wash your hands after applying Desoximetasone Topical Spray, 0.25%.
- Safely throw away (discard) any unused Desoximetasone Topical Spray, 0.25% after 30 days.
See the “Instructions for Use” at the end of the Patient Information for detailed information about the right way to apply Desoximetasone Topical Spray, 0.25%.
What are the possible side effects of Desoximetasone Topical Spray, 0.25%?
Desoximetasone Topical Spray, 0.25% may cause serious side effects, including:
- Desoximetasone Topical Spray, 0.25% can pass through your skin. Too much Desoximetasone Topical Spray, 0.25% passing through your skin can cause your adrenal glands to stop working. Your doctor may do blood tests to check for adrenal gland problems.
The most common side effects of Desoximetasone Topical Spray, 0.25% include dryness, irritation and itching of skin at the treated site.
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of Desoximetasone Topical Spray, 0.25%. For more information, ask your doctor.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
For more information go to fda.report.
How should I store Desoximetasone Topical Spray, 0.25%?
- Store Desoximetasone Topical Spray, 0.25% at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Desoximetasone Topical Spray, 0.25% and all medicines out of the reach of children.
General information about the safe and effective use of Desoximetasone Topical Spray, 0.25%.
- Do not use Desoximetasone Topical Spray, 0.25% for a condition for which it was not prescribed. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Desoximetasone Topical Spray, 0.25% that is written for health professionals.
What are the ingredients in Desoximetasone Topical Spray, 0.25%?
Active ingredient: desoximetasone
Inactive ingredients: glyceryl oleate, isopropyl alcohol, isopropyl myristate, L-menthol, and mineral oil
Made in Israel
Manufactured By Perrigo
Yeruham, Israel
Distributed By
Perrigo®
Allegan, MI 49010 www.perrigo.com
Rev 08-18
- Package/Label Display Panel – 100 mL Carton
-
INGREDIENTS AND APPEARANCE
DESOXIMETASONE
desoximetasone sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 45802-807 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DESOXIMETASONE (UNII: 4E07GXB7AU) (DESOXIMETASONE - UNII:4E07GXB7AU) DESOXIMETASONE 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERYL OLEATE (UNII: 4PC054V79P) ISOPROPYL ALCOHOL (UNII: ND2M416302) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LEVOMENTHOL (UNII: BZ1R15MTK7) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 45802-807-98 1 in 1 CARTON 12/06/2018 1 100 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206441 12/06/2018 Labeler - Perrigo New York Inc (078846912)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.