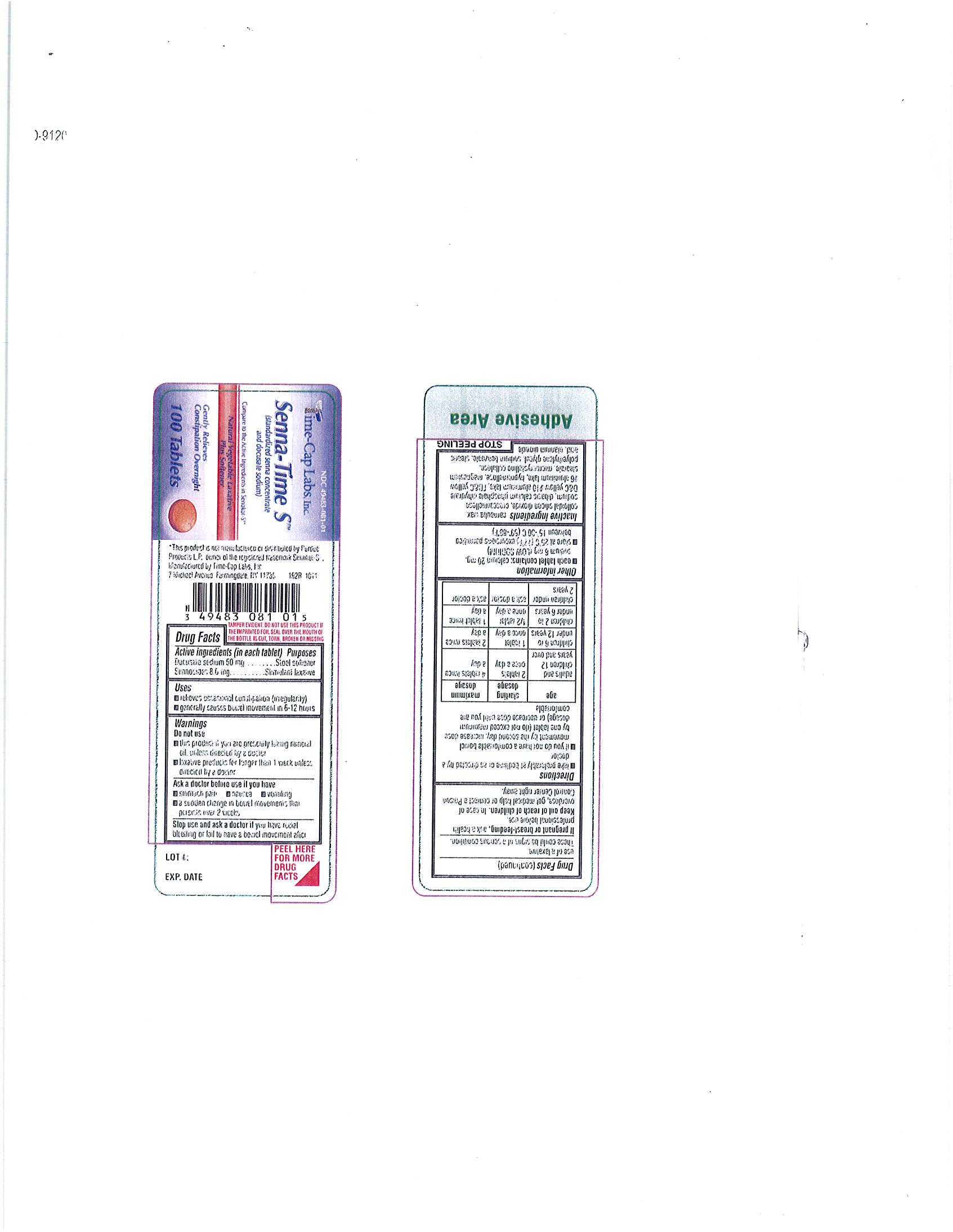
SENNA TIME S- docusate sodium-sennosides tablet, film coated
SENNA TIME S by
Drug Labeling and Warnings
SENNA TIME S by is a Otc medication manufactured, distributed, or labeled by TIME CAP LABORATORIES, INC, TIME CAP LABORATORIES, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS: carnauba wax, colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate dihydrate, d-c yellow#10 aluminum lake fd&c yelow#6 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium benzoate, stearic acid, titanium dioxide - PURPOSE
-
DOSAGE & ADMINISTRATION
Directions:
Take preferably at bedtime or as directed by a doctor. If you do not have a comfortable bowel movement by the second day, increase dose by one tablet (not to exceed maximum dosage) or decrease dose until you are comfortable.
Adults and children 12 years and over - starting dosage: 2 tablets once a day maximum dosage: 4 tablets twice a day
Children 6 to under 12 years - starting dosage: 1 tablet once a day maximum dosage: 2 tablets twice a day
Children to2 to under 6 years - starting dosage: 1/2 tablet once a day maximum dosage: 1 tablet twice a day
Children under 2 years - Ask a doctor
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SENNA TIME S
docusate sodium-sennosides tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49483-081 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg SENNOSIDES A AND B (UNII: 1B5FPI42EN) (SENNOSIDES A AND B - UNII:1B5FPI42EN) SENNOSIDES A AND B 8.6 min Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) SODIUM BENZOATE (UNII: OJ245FE5EU) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape ROUND Size 9mm Flavor Imprint Code TCL081 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49483-081-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/14/2018 2 NDC: 49483-081-10 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/14/2018 3 NDC: 49483-081-00 100000 in 1 CARTON; Type 0: Not a Combination Product 12/14/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 05/14/2012 Labeler - TIME CAP LABORATORIES, INC (037052099) Establishment Name Address ID/FEI Business Operations TIME CAP LABORATORIES, INC. 037052099 manufacture(49483-081)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.