Pain Gel by Good Clean Love, Inc. / TRI-PAC, INC. Pain Gel
Pain Gel by
Drug Labeling and Warnings
Pain Gel by is a Otc medication manufactured, distributed, or labeled by Good Clean Love, Inc., TRI-PAC, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
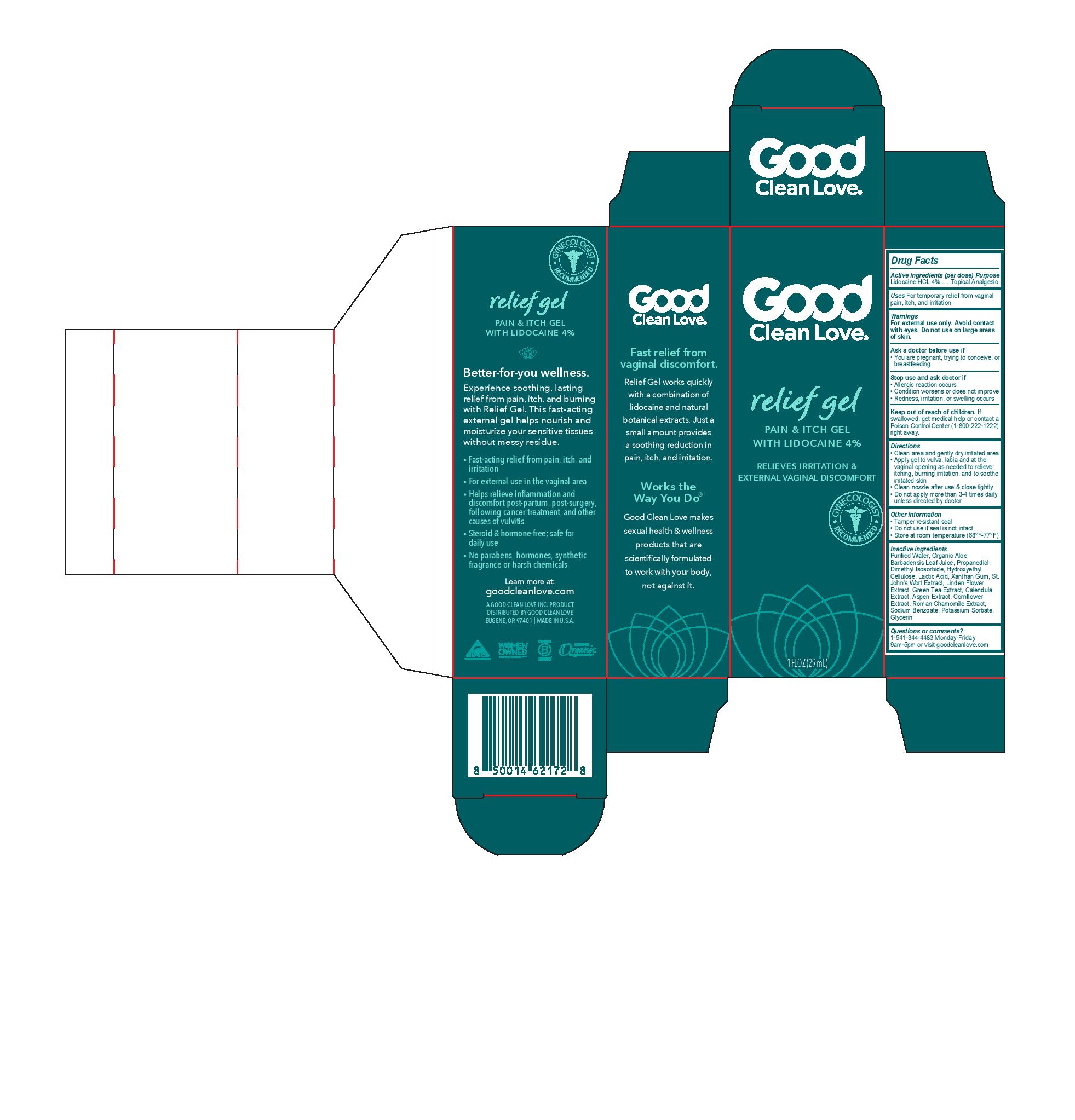
PAIN GEL- lidocaine hcl gel
Good Clean Love, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Pain Gel
Warnings
For external use only. Avoid contact with eyes. Do not use on large areas of skin.
Directions
- Clean area and gently dry irritated area
- Apply gel to vulva, labia and at the vaginal opening as needed to relieve itching, burning irritation, and to soothe irritated skin
- Clean nozzle after use & close tightly
- Do not apply more than 3-4 times daily unless directed by doctor
Other information
- Tamper resistant seal
- Do not use if seal is not intact
- Store at room temperature (68F-77F)
Inactive ingredients
Purified Water, Organic Aloe Barbadensis Leaf Juice, Propanediol, Dimethyl Isosorbide, Hydroxyethyl Cellulose, Lactic Acid, Xanthan Gum, St. John’s Wort Extract, Linden Flower Extract, Green Tea Extract, Calendula Extract, Aspen Extract, Cornflower Extract, Roman Chamomile Extract, Sodium Benzoate, Potassium Sorbate, Glycerin
| PAIN GEL
lidocaine hcl gel |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Good Clean Love, Inc. (153909531) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TRI-PAC, INC. | 020844956 | manufacture(73716-002) | |
Trademark Results [Pain Gel]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PAIN GEL 74493251 not registered Dead/Abandoned |
MENTHOLATUM COMPANY, THE 1994-02-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.