FESOTERODINE FUMARATE tablet, film coated, extended release
FESOTERODINE FUMARATE by
Drug Labeling and Warnings
FESOTERODINE FUMARATE by is a Prescription medication manufactured, distributed, or labeled by Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FESOTERODINE FUMARATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for FESOTERODINE FUMARATE EXTENDED-RELEASE TABLETS.
FESOTERODINE FUMARATE extended-release tablets, for oral use
Initial U.S. Approval: 2008INDICATIONS AND USAGE
Fesoterodine fumarate extended-release tablets are indicated for the treatment of:
DOSAGE AND ADMINISTRATION
- OAB in Adults: The recommended starting dosage is 4 mg orally once daily. Based upon individual response and tolerability, increase to the maximum dosage of 8 mg once daily. (2.1)
-
NDO in Pediatric Patients 6 Years and Older:
- Pediatric Patients Weighing Greater than 25 kg and up to 35 kg:
The recommended dosage is 4 mg orally once daily. If needed, dosage may be increased to 8 mg orally once daily. (2.2)
-
- Pediatric Patients Weighing Greater than 35 kg:
The recommended starting dosage is 4 mg orally once daily. After one week, increase to 8 mg orally once daily. (2.2)
- Adult or Pediatric Patients with Renal Impairment: Refer to the full prescribing information for recommended dosage. (2.3, 2.4)
- Dosage Modifications Due to Strong CYP3A4 Inhibitors: Refer to the full prescribing information for recommended dosage. (2.5)
- Administration: Swallow whole with liquid. Do not chew, divide, or crush. Take with or without food. (2.6)
DOSAGE FORMS AND STRENGTHS
Extended-release tablets: 4 mg and 8 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Angioedema: Promptly discontinue fesoterodine fumarate extended-release tablets and provide appropriate therapy. (5.1)
- Urinary Retention: Fesoterodine fumarate extended-release tablets are not recommended in patients with clinically significant bladder outlet obstruction because of the risk of urinary retention. (5.2)
- Decreased Gastrointestinal Motility: Fesoterodine fumarate extended-release tablets are not recommended for use in patients with decreased gastrointestinal motility, such as those with severe constipation. (5.3)
- Worsening of Narrow-Angle Glaucoma: Use fesoterodine fumarate extended-release tablets with caution in patients being treated for narrow-angle glaucoma. (5.4)
- Central Nervous System Effects: Somnolence has been reported with fesoterodine fumarate extended-release tablets. Advise patients not to drive or operate heavy machinery until they know how fesoterodine fumarate extended-release tablets affect them. (5.5)
- Worsening of Myasthenia Gravis Symptoms: Use fesoterodine fumarate extended-release tablets with caution in patients with myasthenia gravis. (5.6)
ADVERSE REACTIONS
- Most frequently reported adverse events with fesoterodine fumarate extended-release tablets in adult patients with OAB (≥4%) were: dry mouth (placebo, 7%; fesoterodine fumarate extended-release tablets 4 mg, 19%; fesoterodine fumarate extended-release tablets 8 mg, 35%) and constipation (placebo, 2%; fesoterodine fumarate extended-release tablets 4 mg, 4%; fesoterodine fumarate extended-release tablets 8 mg, 6%). (6.1)
- Most frequently reported adverse reactions with fesoterodine fumarate extended-release tablets in pediatric patients (≥2%) with NDO were: diarrhea, urinary tract infection (UTI), dry mouth, constipation, abdominal pain, nausea, weight increased, and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Adult Overactive Bladder
1.2 Pediatric Neurogenic Detrusor Overactivity
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Adult Patients With OAB
2.2 Recommended Dosage for Pediatric Patients Aged 6 Years and Older With NDO
2.3 Recommended Dosage in Adult Patients With Renal Impairment
2.4 Recommended Dosage in Pediatric Patients With Renal Impairment
2.5 Fesoterodine Fumarate Extended-Release Tablets Dosage Modifications Due to Strong CYP3A4 Inhibitors
2.6 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Angioedema
5.2 Urinary Retention in Adult Patients With Bladder Outlet Obstruction
5.3 Decreased Gastrointestinal Motility
5.4 Worsening of Narrow-Angle Glaucoma
5.5 Central Nervous System Effects
5.6 Worsening of Myasthenia Gravis Symptoms
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Antimuscarinic Drugs
7.2 CYP3A4 Inhibitors
7.3 CYP3A4 Inducers
7.4 CYP2D6 Inhibitors
7.5 Drugs Metabolized by Cytochrome P450
7.6 Oral Contraceptives
7.7 Warfarin
7.8 Drug-Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adult Overactive Bladder
14.2 Pediatric Neurogenic Detrusor Overactivity
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Adult Patients With OAB
The recommended starting dosage of fesoterodine fumarate extended-release tablets in adults is 4 mg orally once daily. Based upon individual response and tolerability, increase to the maximum dosage of fesoterodine fumarate extended-release tablets 8 mg once daily. For administration instructions, see Dosage and Administration (2.6).
2.2 Recommended Dosage for Pediatric Patients Aged 6 Years and Older With NDO
Pediatric Patients Weighing Greater than 25 kg and up to 35 kg
The recommended dosage of fesoterodine fumarate extended-release tablets is 4 mg orally once daily. If needed, dosage may be increased to fesoterodine fumarate extended-release tablets 8 mg orally once daily. For administration instructions, see Dosage and Administration (2.6).
Pediatric Patients Weighing Greater than 35 kg
The recommended starting dosage of fesoterodine fumarate extended-release tablets is 4 mg orally once daily. After one week, increase to fesoterodine fumarate extended-release tablets 8 mg orally once daily. For administration instructions, see Dosage and Administration (2.6).2.3 Recommended Dosage in Adult Patients With Renal Impairment
The recommended dosage of fesoterodine fumarate extended-release tablets in adult patients with renal impairment is described in Table 1 [see Use in Specific Populations (8.6)]. For administration instructions, see Dosage and Administration (2.6).
Table 1: Fesoterodine Fumarate Extended-Release Tablets Recommended Dose in Adult Patients With Renal Impairment (Administered Orally Once Daily) 1 Calculate CLcr using the Cockcroft-Gault formula
Estimated Creatinine Clearance1
Recommended Dose
CLcr 30 to 89 mL/min
8 mg
CLcr 15 to 29 mL/min
4 mg
CLcr <15 mL/min
4 mg
2.4 Recommended Dosage in Pediatric Patients With Renal Impairment
Pediatric Patients Weighing Greater than 25 kg and up to 35 kg
The recommended dosage of fesoterodine fumarate extended-release tablets in pediatric patients with renal impairment weighing greater than 25 kg and up to 35 kg is described in Table 2 [see Use in Specific Populations (8.6)]. For administration instructions, see Dosage and Administration (2.6).
Table 2: Fesoterodine Fumarate Extended-Release Tablets Recommended Dose in Pediatric Patients Aged 6 Years and Older Weighing Greater Than 25 kg and up to 35 kg With Renal Impairment (Administered Orally Once Daily) 1 Estimate GFR using a validated GFR estimating equation for the pediatric age range of the approved indication.
2 Dosing was derived assuming similar proportional effects of renal impairment in adults and pediatric patients 6 years and older.
Estimated Glomerular Filtration Rate (GFR)1
Recommended Dose2
eGFR 30 to 89 mL/min/1.73m2
4 mg
eGFR 15 to 29 mL/min/1.73m2
Use is Not Recommended
eGFR <15 mL/min/1.73m2 or requiring dialysis
Use is Not Recommended
Pediatric Patients weighing greater than 35 kg
The recommended dosage of fesoterodine fumarate extended-release tablets in pediatric patients with renal impairment weighing greater than 35 kg is described in Table 3 [see Use in Specific Populations (8.6)]. For administration instructions, see Dosage and Administration (2.6).
Table 3: Fesoterodine Fumarate Extended-Release Tablets Recommended Dose in Pediatric Patients Aged 6 Years and Older Weighing Greater Than 35 kg With Renal Impairment (Administered Orally Once Daily) 1 Estimate GFR using a validated GFR estimating equation for the pediatric age range of the approved indication.
2 The recommended starting dosage of fesoterodine fumarate extended-release tablets is 4 mg orally once daily. After one week, increase to the recommended dosage of fesoterodine fumarate extended-release tablets 8 mg orally once daily.
3 Dosing was derived assuming similar proportional effects of renal impairment in adults and pediatric patients 6 years and older.Estimated GFR1
Recommended Dose3
eGFR 30 to 89 mL/min/1.73m2
8 mg2
eGFR15 to 29 mL/min/1.73m2
4 mg
eGFR <15 mL/min/1.73m2 or requiring dialysis
Use is Not Recommended
2.5 Fesoterodine Fumarate Extended-Release Tablets Dosage Modifications Due to Strong CYP3A4 Inhibitors
Adult Patients with OAB
The maximum recommended dosage is fesoterodine fumarate extended-release tablets 4 mg orally once daily in adult patients taking strong CYP3A4 inhibitors [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)]. For administration instructions, see Dosage and Administration (2.6).
Pediatric Patients with NDO
Pediatric Patients Weighing Greater than 25 kg and up to 35 kg
The use of fesoterodine fumarate extended-release tablets in pediatric patients weighing greater than 25 kg and up to 35 kg and taking strong CYP3A4 inhibitors is not recommended [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)]. For administration instructions, see Dosage and Administration (2.6).
Pediatric Patients Weighing Greater than 35 kg
The maximum recommended dosage is fesoterodine fumarate extended-release tablet 4 mg orally once daily in pediatric patients weighing greater than 35 kg and taking strong CYP3A4 inhibitors [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)]. For administration instructions, see Dosage and Administration (2.6).
2.6 Administration Instructions
Swallow fesoterodine fumarate extended-release tablets whole with liquid. Do not chew, divide, or crush. Take with or without food [see Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Fesoterodine fumarate extended-release tablets are contraindicated in patients with any of the following:
- known or suspected hypersensitivity to fesoterodine fumarate extended-release tablets or any of its ingredients, or to tolterodine tartrate tablets or tolterodine tartrate extended-release capsules [see Clinical Pharmacology (12.1)]. Reactions have included angioedema [see Warnings and Precautions (5.1)]
- urinary retention [see Warnings and Precautions (5.2)]
- gastric retention [see Warnings and Precautions (5.3)]
- uncontrolled narrow-angle glaucoma [see Warnings and Precautions (5.4)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Angioedema
Angioedema of the face, lips, tongue, and/or larynx has been reported with fesoterodine fumarate extended-release tablets. In some cases, angioedema occurred after the first dose; however, cases have been reported to occur hours after the first dose or after multiple doses. Angioedema associated with upper airway swelling may be life-threatening.
Fesoterodine fumarate extended-release tablets are contraindicated in patients with a known or suspected hypersensitivity to fesoterodine fumarate extended-release tablets or any of its ingredients [see Contraindications (4)]. If involvement of the tongue, hypopharynx, or larynx occurs, fesoterodine fumarate extended-release tablets should be promptly discontinued and appropriate therapy and/or measures to ensure a patent airway should be promptly provided.5.2 Urinary Retention in Adult Patients With Bladder Outlet Obstruction
The use of fesoterodine fumarate extended-release tablets, like other antimuscarinic drugs, in patients with clinically significant bladder outlet obstruction, including patients with urinary retention, may result in further urinary retention and kidney injury. The use of fesoterodine fumarate extended-release tablets are not recommended in patients with clinically significant bladder outlet obstruction, and is contraindicated in patients with urinary retention [see Contraindications (4) and Adverse Reactions (6.1)].
5.3 Decreased Gastrointestinal Motility
Fesoterodine fumarate extended-release tablets are associated with decreased gastric motility. Fesoterodine fumarate extended-release tablets are contraindicated in patients with gastric retention [see Contraindications (4)]. The use of fesoterodine fumarate extended-release tablets are not recommended in patients with decreased gastrointestinal motility, such as those with severe constipation.
5.4 Worsening of Narrow-Angle Glaucoma
Fesoterodine fumarate extended-release tablets can worsen controlled narrow-angle glaucoma. Fesoterodine fumarate extended-release tablets are contraindicated in patients with uncontrolled narrow-angle glaucoma [see Contraindications (4)]. Fesoterodine fumarate extended-release tablets should be used with caution in patients being treated for narrow-angle glaucoma.
5.5 Central Nervous System Effects
Fesoterodine fumarate extended-release tablets are associated with anticholinergic central nervous system (CNS) adverse reactions [see Adverse Reactions (6.1)]. A variety of CNS anticholinergic effects have been reported, including headache, dizziness, and somnolence. Patients should be monitored for signs of anticholinergic CNS effects, particularly after beginning treatment or increasing the dose. Advise patients not to drive or operate heavy machinery until they know how fesoterodine fumarate extended-release tablets affect them. If a patient experiences anticholinergic CNS effects, fesoterodine fumarate extended-release tablets dose reduction or discontinuation should be considered.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling:
- Angioedema [see Warnings and Precautions (5.1)]
- Urinary Retention [see Warnings and Precautions (5.2)]
- Decreased Gastrointestinal Motility [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult Overactive Bladder (OAB)
The safety of fesoterodine fumarate extended-release tablets were evaluated in Phase 2 and 3 controlled trials in a total of 2859 patients with overactive bladder, of which 2288 were treated with fesoterodine fumarate extended-release tablets. Of this total, 782 received fesoterodine fumarate extended-release tablets 4 mg/day, and 785 received fesoterodine fumarate extended-release tablets 8 mg/day with treatment periods of 8- or 12-weeks. Approximately 80% of these patients had greater than 10-weeks of exposure to fesoterodine fumarate extended-release tablets in these trials.
A total of 1964 patients participated in two 12-week, Phase 3 efficacy and safety studies and subsequent open-label extension studies. In these two studies combined, 554 patients received fesoterodine fumarate extended-release tablets 4 mg/day and 566 patients received fesoterodine fumarate extended-release tablets 8 mg/day.
In Phase 2 and 3 placebo-controlled trials combined, the incidences of serious adverse events in patients receiving placebo, fesoterodine fumarate extended-release tablets 4 mg, and fesoterodine fumarate extended-release tablets 8 mg were 1.9%, 3.5%, and 2.9%, respectively. All serious adverse events were judged to be not related or unlikely to be related to study medication by the investigator, except for four patients receiving fesoterodine fumarate extended-release tablets who reported one serious adverse reaction each: angina, chest pain, gastroenteritis, and QT prolongation on ECG.
The most commonly reported adverse event in patients treated with fesoterodine fumarate extended-release tablets was dry mouth. The incidence of dry mouth was higher in those taking 8 mg/day (35%) and in those taking 4 mg/day (19%), as compared to placebo (7%). Dry mouth led to discontinuation in 0.4%, 0.4%, and 0.8% of patients receiving placebo, fesoterodine fumarate extended-release tablets 4 mg, and fesoterodine fumarate extended-release tablets 8 mg, respectively. For those patients who reported dry mouth, most had their first occurrence of the event within the first month of treatment.
The second most commonly reported adverse event was constipation. The incidence of constipation was 2% in those taking placebo, 4% in those taking 4 mg/day, and 6% in those taking 8 mg/day.
Table 4 lists adverse events, regardless of causality, that were reported in the combined Phase 3, randomized, placebo-controlled trials at an incidence greater than placebo and in 1% or more of patients treated with fesoterodine fumarate extended-release tablets 4 mg or 8 mg once daily for up to 12-weeks.
Table 4: Adverse Events With an Incidence Exceeding the Placebo Rate and Reported by ≥1% of Patients From Double-Blind, Placebo-Controlled Phase 3 Trials of 12-Weeks Treatment Duration ALT = alanine aminotransferase; GGT = gamma glutamyltransferase System organ class/Preferred term
Placebo
N=554
%
Fesoterodine fumarate extended-release tablets
4 mg/day
N=554
%
Fesoterodine fumarate extended-release tablets
8 mg/day
N=566
%
Gastrointestinal disorders
Dry mouth
Constipation
Dyspepsia
Nausea
7
2
0.5
1.3
18.8
4.2
1.6
0.7
34.6
6
2.3
1.9
Abdominal pain upper
0.5
1.1
0.5
Infections
Urinary tract infection
3.1
3.2
4.2
Upper respiratory tract infection
2.2
2.5
1.8
Eye disorders
Dry eyes
0
1.4
3.7
Renal and urinary disorders
Dysuria
Urinary retention
0.7
0.2
1.3
1.1
1.6
1.4
Respiratory disorders
Cough
Dry throat
0.5
0.4
1.6
0.9
0.9
2.3
General disorders
Edema peripheral
0.7
0.7
1.2
Musculoskeletal disorders
Back pain
0.4
2
0.9
Psychiatric disorders
Insomnia
0.5
1.3
0.4
Investigations
ALT increased
GGT increased
0.9
0.4
0.5
0.4
1.2
1.2
Skin disorders
Rash
0.5
0.7
1.1
Patients also received fesoterodine fumarate extended-release tablets for up to three years in open-label extension phases of one Phase 2 and two Phase 3 controlled trials. In all open-label trials combined, 857, 701, 529, and 105 patients received fesoterodine fumarate extended-release tablets for at least 6 months, 1 year, 2 years, and 3 years, respectively. The adverse events observed during long-term, open-label studies were similar to those observed in the 12-week, placebo-controlled studies, and included dry mouth, constipation, dry eyes, dyspepsia, and abdominal pain. Similar to the controlled studies, most adverse events of dry mouth and constipation were mild to moderate in intensity. Serious adverse events, judged to be at least possibly related to study medication by the investigator and reported more than once during the open-label treatment period of up to 3 years, included urinary retention (3 cases), diverticulitis (3 cases), constipation (2 cases), irritable bowel syndrome (2 cases), and electrocardiogram QT corrected interval prolongation (2 cases).
Pediatric Neurogenic Detrusor Overactivity (NDO)
The safety of fesoterodine fumarate extended-release tablets was evaluated in a total of 131 pediatric patients with NDO. Patients received fesoterodine fumarate extended-release tablets 4 mg or fesoterodine fumarate extended-release tablets 8 mg orally once daily in two clinical trials (Studies 3 and 4).
Study 3 was a Phase 3 study in pediatric patients with NDO from 6 years to 17 years of age and weighing greater than 25 kg. This study consisted of a 12-week efficacy phase, in which 84 patients received fesoterodine fumarate extended-release tablets, followed by a 12-week safety extension phase, in which 103 patients received fesoterodine fumarate extended-release tablets. Of the 103 patients who received fesoterodine fumarate extended-release tablets in the safety extension phase, 67 continued fesoterodine fumarate extended-release tablets from the efficacy phase and 36 switched from an active comparator in the efficacy phase to fesoterodine fumarate extended-release tablets in the safety extension phase.
Study 4 (N=11) was an 8-week, Phase 2 pharmacokinetic (PK) and safety study in pediatric patients with NDO from 8 years to 17 years of age.
The most commonly reported adverse reactions in pediatric patients with NDO who received fesoterodine fumarate extended-release tablets 4 mg or 8 mg in Study 3 (≥2%) were diarrhea, UTI, dry mouth, constipation, abdominal pain, nausea, weight increased and headache.
Table 5 lists the adverse reactions reported at an incidence greater than or equal to 2% in either treatment group in the Study 3 efficacy phase.
Table 5: Adverse Reactions Reported in ≥2% of Patients With NDO Aged 6 Years to 17 Years in the 12-Week Efficacy Phase of Study 3 †Includes abdominal pain and abdominal pain upper Preferred term
Fesoterodine Fumarate Extended-Release Tablets 4 mg
(N=42)
%
Fesoterodine Fumarate Extended-Release Tablets 8 mg
(N=42)
%
Diarrhea
11.9
7.1
Urinary tract infection
9.5
2.4
Dry mouth
7.1
9.5
Constipation
7.1
7.1
Abdominal pain†
7.1
4.8
Nausea
4.8
2.4
Weight increased
4.8
0
Headache
4.8
7.1
Ophthalmological Adverse Reactions
Ophthalmological adverse reactions, including myopia, accommodation disorder and blurred vision, were reported in 8 of 131 (6.1%) pediatric patients with NDO who received fesoterodine fumarate extended-release tablets 4 mg or fesoterodine fumarate extended-release tablets 8 mg in Study 3 (both efficacy and safety extension phases) and Study 4. The ophthalmological adverse reactions did not result in discontinuation of fesoterodine fumarate extended-release tablets in any patient.
Increases in Heart Rate
Increases in heart rate were reported in pediatric patients with NDO who received fesoterodine fumarate extended-release tablets 4 mg and fesoterodine fumarate extended-release tablets 8 mg in Study 3. The mean heart data are described in Table 6.
Table 6: Mean Baseline and Mean Changes From Baseline in Heart Rate in Pediatric Patients Weighing Greater Than 25 kg in Study 3 Study visit
Mean heart rate in beats per minute1 (mean change from baseline)
Fesoterodine Fumarate Extended-Release Tablets 4 mg
Fesoterodine Fumarate Extended-Release Tablets 8 mg
Baseline
88.6
84.2
Week 4
93.8 (+5.2)
94.0 (+9.8)
Week 12
94.8 (+6.2)
94.0 (+9.8)
Week 24
90.4 (+1.8)
90.8 (+6.5)
1 Heart rate expressed as the mean of the baseline measurement and the mean at each study visit and mean changes from baseline at each study visit by original treatment group in patients with complete follow-up at all study visits.
The proportion of patients with heart rates greater than the 99th percentile for age also increased from baseline in patients who received fesoterodine fumarate extended-release tablets 4 mg and fesoterodine fumarate extended-release tablets 8 mg in Study 3. These data are described in Table 7.
Table 7: Proportion of Pediatric Patients With Heart Rate Greater Than the 99th Percentile for Age and Weighing Greater Than 25 kg in Study 3 * Week 12 comprises patients who received fesoterodine fumarate extended-release tablets for 12 weeks after being originally randomized to fesoterodine fumarate extended-release tablets 4 mg and 8 mg and patients originally randomized to active comparator and subsequently transitioned to fesoterodine fumarate extended-release tablets 4 mg and 8 mg for 12 weeks. Study visit
Proportion of patients with heart rate >99th percentile for age
Fesoterodine Fumarate Extended-Release Tablets 4 mg
Fesoterodine Fumarate Extended-Release Tablets 8 mg
Baseline
2.4%
2.4%
Week 4
8.1%
12.2%
Week 12*
7.5%
11.5%
Week 24
3.3%
2.7%
Increases from baseline in the proportion of patients with a heart rate greater than the 99th percentile for age were most pronounced in patients less than 12 years of age who received fesoterodine fumarate extended-release tablets 8 mg.
Increases in heart rate in patients who received fesoterodine fumarate extended-release tablets 4 mg and fesoterodine fumarate extended-release tablets 8 mg in Study 3 were not associated with clinical symptoms and did not result in discontinuation of therapy with fesoterodine fumarate extended-release tablets.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of fesoterodine fumarate extended-release tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac disorders: Palpitations
Central nervous system disorders: Dizziness, headache, somnolence
Eye disorders: Blurred vision
Gastrointestinal disorders: Hypoaesthesia oral
General disorders and administrative site conditions: Hypersensitivity reactions, including angioedema with airway obstruction, face edema
Psychiatric disorders: Confusional state
Skin and subcutaneous tissue disorders: Urticaria, pruritus
-
7 DRUG INTERACTIONS
7.1 Antimuscarinic Drugs
Coadministration of fesoterodine fumarate extended-release tablets with other antimuscarinic agents that produce dry mouth, constipation, urinary retention, and other anticholinergic pharmacological effects may increase the frequency and/or severity of such effects. Anticholinergic agents may potentially alter the absorption of some concomitantly administered drugs due to anticholinergic effects on gastrointestinal motility.
7.2 CYP3A4 Inhibitors
Doses of fesoterodine fumarate extended-release tablets greater than 4 mg are not recommended in adult patients taking strong CYP3A4 inhibitors, such as ketoconazole, itraconazole, and clarithromycin [see Dosage and Administration (2.5)]. The fesoterodine fumarate extended-release tablets dose in pediatric patients taking strong CYP3A4 inhibitors is recommended to be reduced to 4 mg once daily in patients >35 kg and is not recommended in patients weighing greater than 25 kg and up to 35 kg [see Dosage and Administration (2.5)].
In a study in adults, coadministration of the strong CYP3A4 inhibitor ketoconazole with fesoterodine led to approximately a doubling of the maximum concentration (Cmax) and area under the concentration versus time curve (AUC) of 5-hydroxymethyl tolterodine (5-HMT), the active metabolite of fesoterodine. Compared with CYP2D6 extensive metabolizers not taking ketoconazole, further increases in the exposure to 5-HMT were observed in subjects who were CYP2D6 poor metabolizers taking ketoconazole [see Clinical Pharmacology (12.3)].
There is no clinically relevant effect of moderate CYP3A4 inhibitors on the pharmacokinetics of fesoterodine. Following blockade of CYP3A4 by coadministration of the moderate CYP3A4 inhibitor fluconazole 200 mg twice a day for 2 days, the average (90% confidence interval) increase in Cmax and AUC of the active metabolite of fesoterodine was approximately 19% (11% to 28%) and 27% (18% to 36%) respectively. No dosing adjustments are recommended in the presence of moderate CYP3A4 inhibitors (e.g., erythromycin, fluconazole, diltiazem, verapamil and grapefruit juice).
The effect of weak CYP3A4 inhibitors (e.g., cimetidine) was not examined; it is not expected to be in excess of the effect of moderate inhibitors [see Clinical Pharmacology (12.3)].
7.3 CYP3A4 Inducers
No dosing adjustments are recommended in the presence of CYP3A4 inducers, such as rifampin and carbamazepine. Following induction of CYP3A4 by coadministration of rifampin 600 mg once a day, Cmax and AUC of the active metabolite of fesoterodine decreased by approximately 70% and 75%, respectively, after oral administration of fesoterodine fumarate extended-release tablets 8 mg. The terminal half-life of the active metabolite was not changed.
7.4 CYP2D6 Inhibitors
The interaction with CYP2D6 inhibitors was not tested clinically. In poor metabolizers for CYP2D6, representing a maximum CYP2D6 inhibition, Cmax and AUC of the active metabolite are increased 1.7- and 2-fold, respectively.
No dosing adjustments are recommended in the presence of CYP2D6 inhibitors.
7.5 Drugs Metabolized by Cytochrome P450
In vitro data indicate that at therapeutic concentrations, the active metabolite of fesoterodine does not have the potential to inhibit or induce Cytochrome P450 enzyme systems [see Clinical Pharmacology (12.3)].
7.6 Oral Contraceptives
In the presence of fesoterodine, there are no clinically significant changes in the plasma concentrations of combined oral contraceptives containing ethinyl estradiol and levonorgestrel [see Clinical Pharmacology (12.3)].
7.7 Warfarin
A clinical study has shown that fesoterodine 8 mg once daily has no significant effect on the pharmacokinetics or the anticoagulant activity (PT/INR) of warfarin 25 mg. Standard therapeutic monitoring for warfarin should be continued [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with the use of fesoterodine fumarate extended-release tablets in pregnant women and adolescents to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of fesoterodine to pregnant mice and rabbits during organogenesis resulted in fetotoxicity at maternal exposures that were 6 and 3 times respectively the maximum recommended human dose (MRHD) of 8 mg/day, based on AUC (see Data). The background risk of major birth defects and miscarriage for the indicated population are unknown. However, in the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
No dose-related teratogenicity was observed in reproduction studies performed in mice and rabbits. In mice at 6 to 27 times the expected exposure at the maximum recommended human dose (MRHD) of 8 mg based on AUC (75 mg/kg/day, oral), increased resorptions and decreased live fetuses were observed. One fetus with cleft palate was observed at each dose (15, 45, and 75 mg/kg/day), at an incidence within the background historical range. In rabbits treated at 3 to 11 times the MRHD (27 mg/kg/day, oral), incompletely ossified sternebrae (retardation of bone development) and reduced survival were observed in fetuses. In rabbits at 9 to 11 times the MRHD (4.5 mg/kg/day, subcutaneous), maternal toxicity and incompletely ossified sternebrae were observed in fetuses (at an incidence within the background historical range). In rabbits at 3 times the MRHD (1.5 mg/kg/day, subcutaneous), decreased maternal food consumption in the absence of any fetal effects was observed. Oral administration of 30 mg/kg/day fesoterodine to mice in a pre- and post-natal development study resulted in decreased body weight of the dams and delayed ear opening of the pups. No effects were noted on mating and reproduction of the F1 dams or on the F2 offspring.8.2 Lactation
Risk Summary
There is no information on the presence of fesoterodine in human milk, the effects on the breastfed child, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for fesoterodine fumarate extended-release tablets and any potential adverse effects on the breastfed child from fesoterodine fumarate extended-release tablets or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of fesoterodine fumarate extended-release tablets have been established for the treatment of neurogenic detrusor overactivity (NDO) in pediatric patients aged 6 years and older and weighing greater than 25 kg. The information on this use is discussed throughout labeling. Use of fesoterodine fumarate extended-release tablets for treatment of NDO is supported by evidence from a randomized, open-label trial with an initial 12-week efficacy phase followed by a 12-week safety extension phase in pediatric patients from 6 years to 17 years of age (Study 3) [see Adverse Reactions (6.1) and Clinical Studies (14.2)]. Study results demonstrated that treatment with fesoterodine fumarate extended-release tablets 4 mg and 8 mg daily resulted in improvements from baseline to Week 12 in maximum cystometric bladder capacity (MCBC) for patients weighing greater than 25 kg [see Clinical Studies (14.2) and Clinical Pharmacology (12.3)]. The most commonly reported adverse reactions in patients who received fesoterodine fumarate extended-release tablets 4 mg or 8 mg in Study 3 (≥2%) were diarrhea, UTI, dry mouth, constipation, abdominal pain, nausea, weight increase and headache [see Adverse Reactions (6.1)]. Mean increases from baseline in heart rate were reported with both the 4 mg and 8 mg daily doses of fesoterodine fumarate extended-release tablets, with larger mean increases reported in pediatric patients who received the 8 mg daily dose [see Adverse Reactions (6.1)].
The safety and effectiveness of fesoterodine fumarate extended-release tablets have not been established in pediatric patients younger than 6 years of age or weighing 25 kg or less.
8.5 Geriatric Use
No dose adjustment is recommended for the elderly. The pharmacokinetics of fesoterodine are not significantly influenced by age.
Of the 1,567 patients who received fesoterodine fumarate extended-release tablets 4 mg or 8 mg orally once daily in Phase 2 and 3, placebo-controlled, efficacy and safety studies for OAB, 515 (33%) were 65 years of age or older, and 140 (9%) were 75 years of age or older. No overall difference in effectiveness was observed between patients younger than 65 years of age and those 65 years of age or older in these studies. However, the incidence of antimuscarinic adverse reactions, including dry mouth, constipation, dyspepsia, increase in residual urine, dizziness (8 mg only) and urinary tract infection, was higher in patients 75 years of age and older as compared to younger patients [see Clinical Studies (14.1) and Adverse Reactions (6)].
8.6 Renal Impairment
In adult patients with severe renal impairment (CLCR <30 mL/min), Cmax and AUC are increased 2- and 2.3-fold, respectively. Doses of fesoterodine fumarate extended-release tablets greater than 4 mg are not recommended in adult patients with severe renal impairment. In patients with mild or moderate renal impairment (CLCR ranging from 30 to 80 mL/min), Cmax and AUC of the active metabolite are increased up to 1.5- and 1.8-fold, respectively, as compared to healthy subjects. No dose adjustment is recommended in patients with mild or moderate renal impairment [see Clinical Pharmacology (12.3) and Dosage and Administration (2.2, 2.3)].
The recommended dosage of fesoterodine fumarate extended-release tablets in pediatric patients weighing greater than 25 kg and up to 35 kg with mild-to-moderate renal impairment (eGFR 30 to 89 mL/min/1.73m2) is 4 mg once daily and fesoterodine fumarate extended-release tablets is not recommend in those with severe renal impairment (eGFR 15 to 29 mL/min/1.73m2). In pediatric patients weighing greater than 35 kg with mild-to-moderate renal impairment (eGFR 30 to 89 mL/min/1.73m2), the recommended starting dosage of fesoterodine fumarate extended-release tablets is 4 mg orally once daily, with increase to the recommended dosage of fesoterodine fumarate extended-release tablets 8 mg orally once daily, and in those with severe renal impairment (eGFR 15 to 29 mL/min/1.73m2) the recommended dose is 4 mg once daily [see Dosage and Administration (2.2, 2.4)].8.7 Hepatic Impairment
Patients with severe hepatic impairment (Child-Pugh C) have not been studied; therefore fesoterodine fumarate extended-release tablets are not recommended for use in these patients. In patients with moderate (Child-Pugh B) hepatic impairment, Cmax and AUC of the active metabolite are increased 1.4- and 2.1-fold, respectively, as compared to healthy subjects. No dose adjustment is recommended in patients with mild or moderate hepatic impairment [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Fesoterodine fumarate extended-release tablet contains fesoterodine fumarate. Fesoterodine is rapidly de-esterified to its active metabolite (R)-2-(3-diisopropylamino-1-phenylpropyl)-4-hydroxymethyl-phenol, or 5-hydroxymethyl tolterodine, which is a muscarinic receptor antagonist.
Chemically, fesoterodine fumarate is designated as isobutyric acid 2-((R)-3-diisopropylammonium-1-phenylpropyl)-4-(hydroxymethyl) phenyl ester hydrogen fumarate. The molecular formula is C30H41NO7 and its molecular weight is 527.66. The structural formula is:
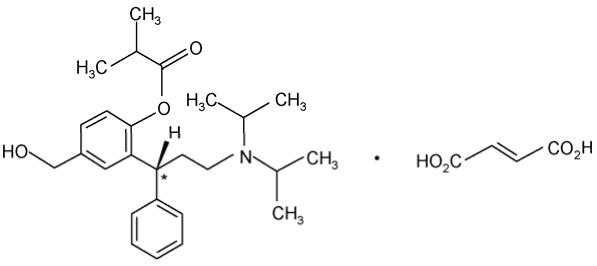
The asterisk (*) indicates the chiral carbon.
Fesoterodine fumarate is a white to off-white powder, which is freely soluble in water. Each fesoterodine fumarate extended-release tablet contains either 4 mg or 8 mg of fesoterodine fumarate and the following inactive ingredients: FD&C Blue #2/indigo carmine aluminum lake, glyceryl dibehenate, hypromellose, lactose monohydrate, lecithin (soya), microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and xylitol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fesoterodine is a competitive muscarinic receptor antagonist. After oral administration, fesoterodine is rapidly and extensively hydrolyzed by nonspecific esterases to its active metabolite, 5-hydroxymethyl tolterodine, which is responsible for the antimuscarinic activity of fesoterodine.
Muscarinic receptors play a role in contractions of urinary bladder smooth muscle. Inhibition of these receptors in the bladder is presumed to be the mechanism by which fesoterodine produces its effects.12.2 Pharmacodynamics
In a urodynamic study involving patients with involuntary detrusor contractions, the effects after the administration of fesoterodine on the volume at first detrusor contraction and bladder capacity were assessed. Administration of fesoterodine increased the volume at first detrusor contraction and bladder capacity in a dose-dependent manner. These findings are consistent with an antimuscarinic effect on the bladder.
Cardiac Electrophysiology
The effect of fesoterodine 4 mg and 28 mg on the QT interval was evaluated in a double-blind, randomized, placebo- and positive-controlled (moxifloxacin 400 mg once a day) parallel trial with once-daily treatment over a period of 3 days in 261 male and female subjects aged 44 to 65 years. Electrocardiographic parameters were measured over a 24-hour period at pre-dose, after the first administration, and after the third administration of study medication. Fesoterodine 28 mg was chosen because this dose, when administered to CYP2D6 extensive metabolizers, results in an exposure to the active metabolite that is similar to the exposure in a CYP2D6 poor metabolizer receiving fesoterodine 8 mg together with CYP3A4 blockade. Corrected QT intervals (QTc) were calculated using Fridericia’s correction and a linear individual correction method. Analyses of 24-hour average QTc, time-matched baseline-corrected QTc, and time-matched placebo-subtracted QTc intervals indicate that fesoterodine at doses of 4 and 28 mg/day did not prolong the QT interval. The sensitivity of the study was confirmed by positive QTc prolongation by moxifloxacin.
In this study, conducted in subjects aged 44 to 65 years, fesoterodine fumarate extended-release tablets were associated with an increase in heart rate that correlates with increasing dose. When compared to placebo, the mean increase in heart rate associated with fesoterodine 4 mg/day and fesoterodine 28 mg/day was 3 beats/minute and 11 beats/minute, respectively.
In the two, phase 3, placebo-controlled studies in adult in patients with overactive bladder, the mean increases in heart rate compared to placebo were 3 to 4 beats/minute in the fesoterodine 4 mg/day group and 3 to 5 beats/minute in the fesoterodine 8 mg/day group.
12.3 Pharmacokinetics
Absorption
After oral administration, fesoterodine is well absorbed. Due to rapid and extensive hydrolysis by nonspecific esterases to its active metabolite 5-hydroxymethyl tolterodine, fesoterodine cannot be detected in plasma. Bioavailability of the active metabolite is 52%. After single or multiple-dose oral administration of fesoterodine in doses from 4 mg to 28 mg, plasma concentrations of the active metabolite are proportional to the dose. Maximum plasma levels are reached after approximately 5 hours. No accumulation occurs after multiple-dose administration.
A Summary of pharmacokinetic parameters for the active metabolite after a single dose of fesoterodine fumarate extended-release tablets 4 mg and 8 mg in extensive and poor metabolizers of CYP2D6 is provided in Table 8.Table 8: Summary of Geometric Mean [CV] Pharmacokinetic Parameters for the Active Metabolite After a Single Dose of Fesoterodine Fumarate Extended-Release Tablets 4 mg and 8 mg in Extensive and Poor CYP2D6 Metabolizers EM = extensive CYP2D6 metabolizer, PM = poor CYP2D6 metabolizer, CV = coefficient of variation; Cmax = maximum plasma concentration, AUC0-tz = area under the concentration time curve from zero up to the last measurable plasma concentration, tmax = time to reach Cmax, t½ = terminal half-life
aData presented as median (range)
Fesoterodine fumarate extended-release tablets
4 mg
Fesoterodine fumarate extended-release tablets
8 mg
Parameter
EM (N=16)
PM (N=8)
EM (N=16)
PM (N=8)
Cmax (ng/mL)
1.89 [43%]
3.45 [54%]
3.98 [28%]
6.9 [39%]
AUC0-tz (ng*h/mL)
21.2 [38%]
40.5 [31%]
45.3 [32%]
88.7 [36%]
tmax (h)a
5 [2-6]
5 [5-6]
5 [3-6]
5 [5-6]
t½ (h)
7.31 [27%]
7.31 [30%]
8.59 [41%]
7.66 [21%]
Effect of Food
There is no clinically relevant effect of food on the pharmacokinetics of fesoterodine. In a study of the effects of food on the pharmacokinetics of fesoterodine in 16 healthy male volunteers, concomitant food intake increased the active metabolite of fesoterodine AUC by approximately 19% and Cmax by 18% [see Dosage and Administration (2.1)].Distribution
Plasma protein binding of the active metabolite is low (approximately 50%) and is primarily bound to albumin and alpha-1-acid glycoprotein. The mean steady-state volume of distribution following intravenous infusion of the active metabolite is 169 L.
Metabolism
After oral administration, fesoterodine is rapidly and extensively hydrolyzed to its active metabolite. The active metabolite is further metabolized in the liver to its carboxy, carboxy-N-desisopropyl, and N-desisopropyl metabolites via two major pathways involving CYP2D6 and CYP3A4. None of these metabolites contribute significantly to the antimuscarinic activity of fesoterodine.
Variability in CYP2D6 Metabolism
A subset of individuals (approximately 7% of Caucasians and approximately 2% of African Americans) are poor metabolizers for CYP2D6. Cmax and AUC of the active metabolite are increased 1.7- and 2-fold, respectively, in CYP2D6 poor metabolizers, as compared to extensive metabolizers.
Excretion
Hepatic metabolism and renal excretion contribute significantly to the elimination of the active metabolite. After oral administration of fesoterodine, approximately 70% of the administered dose was recovered in urine as the active metabolite (16%), carboxy metabolite (34%), carboxy-N-desisopropyl metabolite (18%), or N-desisopropyl metabolite (1%), and a smaller amount (7%) was recovered in feces.
The terminal half-life of the active metabolite is approximately 4 hours following an intravenous administration. The apparent terminal half-life following oral administration is approximately 7 hours.
Pharmacokinetics in Specific Populations
Geriatric PatientsFollowing a single 8 mg oral dose of fesoterodine, the mean (±SD) AUC and Cmax for the active metabolite 5-hydroxymethyl tolterodine in 12 elderly men (mean age 67 years) were 51.8 ± 26.1 h*ng/mL and 3.8 ± 1.7 ng/mL, respectively. In the same study, the mean (±SD) AUC and Cmax in 12 young men (mean age 30 years) were 52 ± 31.5 h*ng/mL and 4.1 ± 2.1 ng/mL, respectively. The pharmacokinetics of fesoterodine were not significantly influenced by age [see Use in Specific Populations (8.5)].
Pediatric Patients
In pediatric patients, from 6 years to 17 years of age with NDO weighing 35 kg with CYP2D6 extensive metabolizer status receiving fesoterodine fumarate extended-release tablets, the mean values of apparent oral clearance, volume of distribution and absorption rate constant of 5-HMT are estimated to be approximately 72 L/h, 68 L and 0.09 h-1, respectively. The Tmax and half-life of 5-HMT are estimated to be approximately 2.55 h and 7.73 h, respectively. Like adults, the 5-HMT exposures in CYP2D6 poor metabolizers was estimated to be approximately 2-fold higher compared with extensive metabolizers.
The post-hoc estimates of steady-state exposures of 5-HMT in NDO patients weighing greater than 25 kg following fesoterodine fumarate extended-release tablets 4 mg and 8 mg once daily are summarized in Table 9.
Table 9: Summary of Geometric Mean [%CV] Pharmacokinetic Parameters for the Active Metabolite After Steady-State Dosing of Fesoterodine in Pediatric Patients With NDO, Ages 6 to 17 Years Weighing Greater Than 25 kg CV = coefficient of variation; Cmax,ss = steady-state maximum plasma concentration, AUCtau,ss = steady-state area under the concentration time curve over the 24-hour dosing interval, N = number of patients with PK data Dosage
N
Cmax,ss (ng/mL)
AUCtau,ss (ng*h/mL)
4 mg once daily
32
4.88 (48.2)
59.1 (51.7)
8 mg once daily
39
8.47 (41.6)
103 (46.2)
Gender
Following a single 8 mg oral dose of fesoterodine, the mean (±SD) AUC and Cmax for the active metabolite 5-hydroxymethyl tolterodine in 12 elderly men (mean age 67 years) were 51.8 ± 26.1 h*ng/mL and 3.8 ± 1.7 ng/mL, respectively. In the same study, the mean (±SD) AUC and Cmax in 12 elderly women (mean age 68 years) were 56 ± 28.8 h*ng/mL and 4.6 ± 2.3 ng/mL, respectively. The pharmacokinetics of fesoterodine were not significantly influenced by gender.
Race
The effects of Caucasian or Black race on the pharmacokinetics of fesoterodine were examined in a study of 12 Caucasian and 12 Black African young male volunteers. Each subject received a single oral dose of 8 mg fesoterodine. The mean (±SD) AUC and Cmax for the active metabolite 5-hydroxymethyl tolterodine in Caucasian males were 73 ± 27.8 h*ng/mL and 6.1 ± 2.7 ng/mL, respectively. The mean (±SD) AUC and Cmax in Black males were 65.8 ± 23.2 h*ng/mL and 5.5 ± 1.9 ng/mL, respectively. The pharmacokinetics of fesoterodine were not significantly influenced by race.
Renal Impairment
In patients with mild or moderate renal impairment (CLCR ranging from 30 to 80 mL/min), Cmax and AUC of the active metabolite are increased up to 1.5- and 1.8-fold, respectively, as compared to healthy subjects. In patients with severe renal impairment (CLCR <30 mL/min), Cmax and AUC are increased 2- and 2.3-fold, respectively [see Use in Specific Populations (8.6) and Dosage and Administration (2.2, 2.3)].
Hepatic Impairment
In patients with moderate (Child-Pugh B) hepatic impairment, Cmax and AUC of the active metabolite are increased 1.4- and 2.1-fold, respectively, as compared to healthy subjects.
Subjects with severe hepatic impairment (Child-Pugh C) have not been studied [see Use in Specific Populations (8.7)].
Drug-Drug Interactions
Drugs Metabolized by Cytochrome P450
At therapeutic concentrations, the active metabolite of fesoterodine does not inhibit CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, or 3A4, or induce CYP1A2, 2B6, 2C9, 2C19, or 3A4 in vitro [see Drug Interactions (7.5)].
CYP3A4 Inhibitors
Following blockade of CYP3A4 by coadministration of the strong CYP3A4 inhibitor ketoconazole 200 mg twice a day for 5 days, Cmax and AUC of the active metabolite of fesoterodine increased 2- and 2.3-fold, respectively, after oral administration of fesoterodine fumarate extended-release tablets 8 mg to CYP2D6 extensive metabolizers. In CYP2D6 poor metabolizers, Cmax and AUC of the active metabolite of fesoterodine increased 2.1- and 2.5-fold, respectively, during coadministration of ketoconazole 200 mg twice a day for 5 days. Cmax and AUC were 4.5-and 5.7-fold higher, respectively, in subjects who were CYP2D6 poor metabolizers and taking ketoconazole compared to subjects who were CYP2D6 extensive metabolizers and not taking ketoconazole. In a separate study coadministering fesoterodine with ketoconazole 200 mg once a day for 5 days, the Cmax and AUC values of the active metabolite of fesoterodine were increased 2.2-fold in CYP2D6 extensive metabolizers and 1.5- and 1.9-fold, respectively, in CYP2D6 poor metabolizers. Cmax and AUC were 3.4- and 4.2-fold higher, respectively, in subjects who were CYP2D6 poor metabolizers and taking ketoconazole compared to subjects who were CYP2D6 extensive metabolizers and not taking ketoconazole.
There is no clinically relevant effect of moderate CYP3A4 inhibitors on the pharmacokinetics of fesoterodine. In a drug-drug interaction study evaluating the coadministration of the moderate CYP3A4 inhibitor fluconazole 200 mg twice a day for 2 days, a single 8 mg dose of fesoterodine was administered 1 hour following the first dose of fluconazole on day 1 of the study. The average (90% confidence interval) for the increase in Cmax and AUC of the active metabolite of fesoterodine was approximately 19% (11% to 28%) and 27% (18% to 36%) respectively.
The effect of weak CYP3A4 inhibitors (e.g., cimetidine) was not examined; it is not expected to be in excess of the effect of moderate inhibitors [see Drug Interactions (7.2) and Dosage and Administration (2.2, 2.3)].
CYP3A4 Inducers
Following induction of CYP3A4 by coadministration of rifampicin 600 mg once a day, Cmax and AUC of the active metabolite of fesoterodine decreased by approximately 70% and 75%, respectively, after oral administration of fesoterodine fumarate extended-release tablets 8 mg. The terminal half-life of the active metabolite was not changed.
Induction of CYP3A4 may lead to reduced plasma levels. No dosing adjustments are recommended in the presence of CYP3A4 inducers [see Drug Interactions (7.3)].
CYP2D6 Inhibitors
The interaction with CYP2D6 inhibitors was not studied. In poor metabolizers for CYP2D6, representing a maximum CYP2D6 inhibition, Cmax and AUC of the active metabolite are increased 1.7- and 2-fold, respectively [see Drug Interactions (7.4)].
Oral Contraceptives
Thirty healthy female subjects taking an oral contraceptive containing 0.03 mg ethinyl estradiol and 0.15 mg levonorgestrel were evaluated in a 2-period cross-over study. Each subject was randomized to receive concomitant administration of either placebo or fesoterodine 8 mg once daily on days 1 to 14 of hormone cycle for 2 consecutive cycles. Pharmacokinetics of ethinyl estradiol and levonorgestrel were assessed on day 13 of each cycle. Fesoterodine increased the AUC and Cmax of ethinyl estradiol by 1 to 3% and decreased the AUC and Cmax of levonorgestrel by 11 to 13% [see Drug Interactions (7.6)].
Warfarin
In a cross-over study in 14 healthy male volunteers (18 to 55 years), a single oral dose of warfarin 25 mg was given either alone or on day 3 of once daily dosing for 9 days with fesoterodine 8 mg. Compared to warfarin alone dosing, the Cmax and AUC of S-warfarin were lower by ~ 4 %, while the Cmax and AUC of R-warfarin were lower by approximately 8 % and 6% for the coadministration, suggesting absence of a significant pharmacokinetic interaction.
There were no statistically significant changes in the measured pharmacodynamic parameters for anticoagulant activity of warfarin (INRmax, AUCINR), with only a small decrease noted in INRmax of ~ 3 % with the co-administration relative to warfarin alone. INR versus time profiles across individual subjects in the study suggested some differences following co-administration with fesoterodine, although there was no definite trend with regard to the changes noted [see Drug Interactions (7.7)]. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
No evidence of drug-related carcinogenicity was found in 24-month studies with oral administration to mice and rats. The highest tolerated doses in mice (females 45 to 60 mg/kg/day, males 30 to 45 mg/kg/day) correspond to 11 to 19 times (females) and 4 to 9 times (males) the estimated human AUC values reached with fesoterodine 8 mg, which is the Maximum Recommended Human Dose (MRHD). In rats, the highest tolerated dose (45 to 60 mg/kg/day) corresponds to 3 to 8 times (females) and 3 to 14 times (males) the estimated human AUC at the MRHD.
Mutagenesis
Fesoterodine was not mutagenic or genotoxic in vitro (Ames tests, chromosome aberration tests) or in vivo (mouse micronucleus test).
Impairment of Fertility
Fesoterodine had no effect on male reproductive function or fertility at doses up to 45 mg/kg/day in mice. At 45 mg/kg/day, a lower number of corpora lutea, implantation sites and viable fetuses was observed in female mice administered fesoterodine for 2-weeks prior to mating and continuing through day 7 of gestation. The maternal No-Observed-Effect Level (NOEL) and the NOEL for effects on reproduction and early embryonic development were both 15 mg/kg/day. At the NOEL, the systemic exposure, based on AUC, was 0.6 to 1.5 times higher in mice than in humans at the MRHD, whereas based on peak plasma concentrations, the exposure in mice was 5 to 9 times higher.
-
14 CLINICAL STUDIES
14.1 Adult Overactive Bladder
The efficacy of fesoterodine fumarate extended-release tablets was evaluated in two, Phase 3, randomized, double-blind, placebo-controlled, 12-week studies for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency. Entry criteria required that patients have symptoms of overactive bladder for ≥6-months duration, at least 8 micturitions per day, and at least 6 urinary urgency episodes or 3 urge incontinence episodes per 3-day diary period. Patients were randomized to a fixed dose of fesoterodine fumarate extended-release tablets 4 or 8 mg/day or placebo. In one of these studies, 290 patients were randomized to an active control arm (an oral antimuscarinic agent). For the combined studies, a total of 554 patients received placebo, 554 patients received fesoterodine fumarate extended-release tablets 4 mg/day, and 566 patients received fesoterodine fumarate extended-release tablets 8 mg/day. The majority of patients were Caucasian (91%) and female (79%) with a mean age of 58 years (range 19 to 91 years).
The primary efficacy endpoints were the mean change in the number of urge urinary incontinence episodes per 24 hours and the mean change in the number of micturitions (frequency) per 24 hours. An important secondary endpoint was the mean change in the voided volume per micturition.
Results for the primary endpoints and for mean change in voided volume per micturition from the two 12-week clinical studies of fesoterodine fumarate extended-release tablets are reported in Table 10.
Table 10: Mean Baseline and Change From Baseline to Week 12 for Urge Urinary Incontinence Episodes, Number of Micturitions, and Volume Voided per Micturition
Study 1
Study 2
Parameter
Placebo
N=279
Fesoterodine fumarate extended-release tablets
4 mg/day
N=265
Fesoterodine fumarate extended-release tablets
8 mg/day
N=276
Placebo
N=266
Fesoterodine fumarate extended-release tablets
4 mg/day
N=267
Fesoterodine fumarate extended-release tablets
8 mg/day
N=267
Number of urge incontinence episodes per 24 hoursa
Baseline
3.7
3.8
3.7
3.7
3.9
3.9
Change from baseline
-1.2
-2.06
-2.27
-1
-1.77
-2.42
p-value vs. placebo
-
0.001
<0.001
-
<0.003
<0.001
Number of micturitions per 24 hours
Baseline
12
11.6
11.9
12.2
12.9
12
Change from baseline
-1.02
-1.74
-1.94
-1.02
-1.86
-1.94
p-value vs. placebo
-
<0.001
<0.001
-
0.032
<0.001
Voided volume per micturition (mL)
Baseline
150
160
154
159
152
156
Change from baseline
10
27
33
8
17
33
p-value vs. placebo
-
<0.001
<0.001
-
0.15
<0.001
vs. = versus
a Only those patients who were urge incontinent at baseline were included for the analysis of number of urge incontinence episodes per 24 hours: In Study 1, the number of these patients was 211, 199, and 223 in the placebo, fesoterodine fumarate extended-release tablets 4 mg/day and fesoterodine fumarate extended-release tablets 8 mg/day groups, respectively. In Study 2, the number of these patients was 205, 228, and 218, respectively.
Figures 1 to 4: The following figures show change from baseline over time in number of micturitions and urge urinary incontinence episodes per 24 h in the two studies.
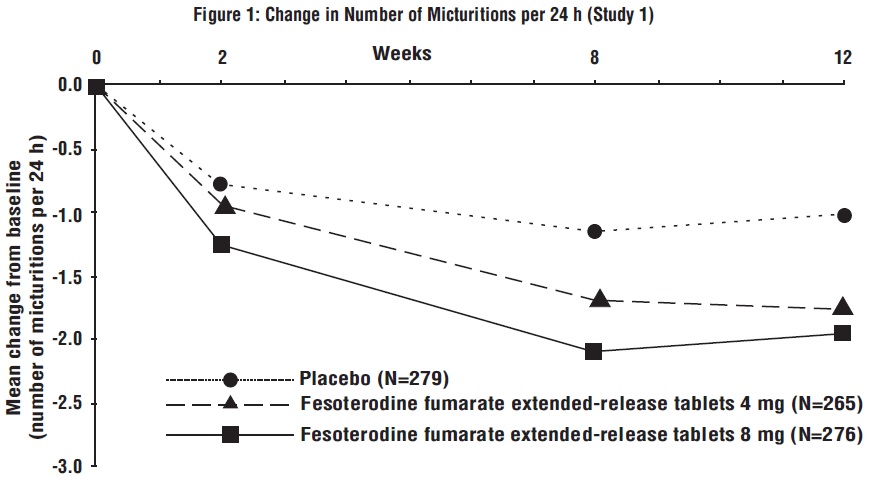
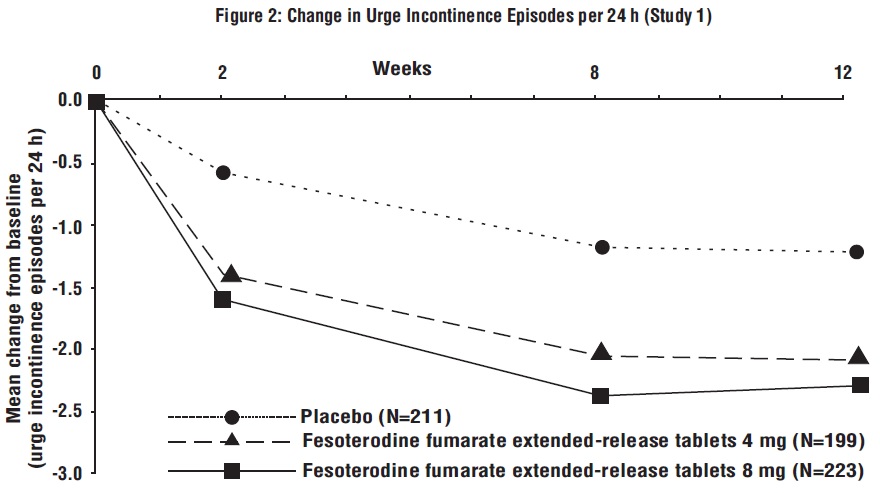
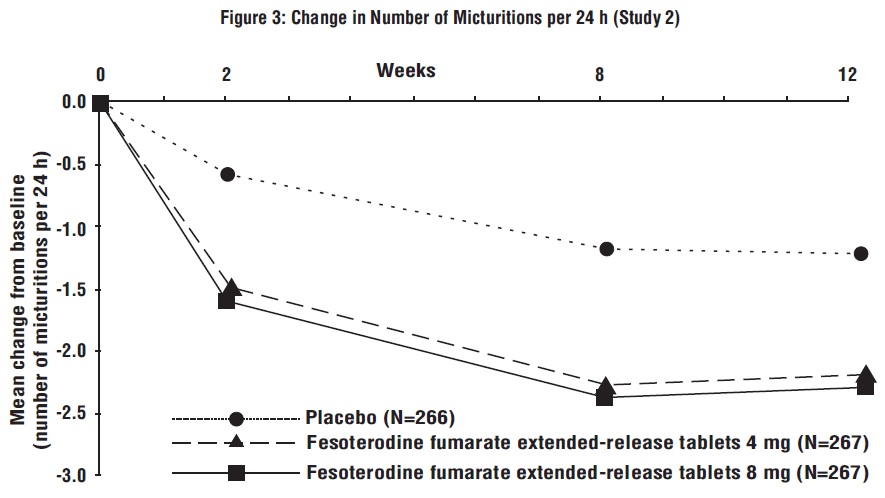
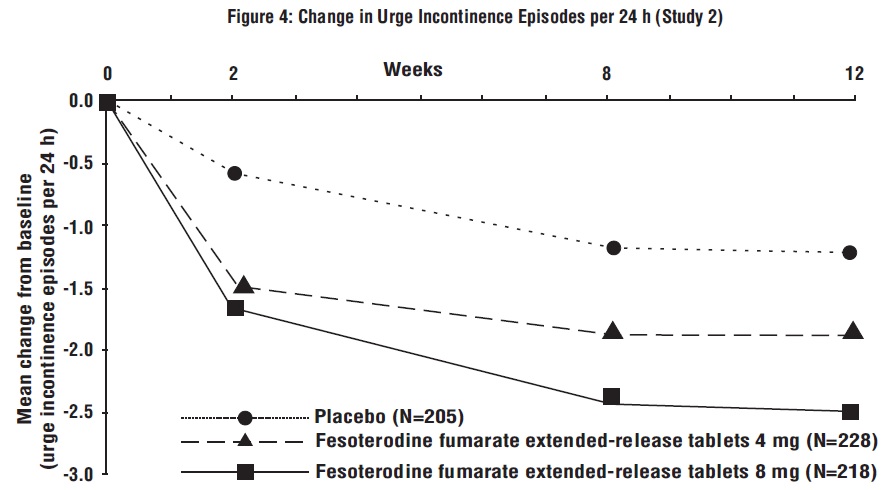
A reduction in number of urge urinary incontinence episodes per 24 hours was observed for both doses as compared to placebo as early as two weeks after starting fesoterodine fumarate extended-release tablets therapy.
14.2 Pediatric Neurogenic Detrusor Overactivity
The efficacy of fesoterodine fumarate extended-release tablets was evaluated in Study 3 (NCT01557244), a Phase 3, randomized, open-label study consisting of a 12-week efficacy phase followed by a 12-week safety extension phase in pediatric patients from 6 years to 17 years of age. Two cohorts were studied. Cohort 1 (patients weighing greater than 25 kg) received a fixed dose of fesoterodine fumarate extended-release tablets 4 mg or fesoterodine fumarate extended-release tablets 8 mg orally once daily, or once daily. In the safety extension phase, patients randomized to the active comparator were switched to fesoterodine fumarate extended-release tablets 4 mg or fesoterodine fumarate extended-release tablets 8 mg once daily. For study inclusion, patients were required to have stable neurological disease and clinically or urodynamically-demonstrated NDO. Cohort 2 patients weighing less than 25 kg received an investigational fesoterodine formulation.
During the 12-week efficacy phase, 124 patients (69 males and 55 females) were randomized to receive fesoterodine fumarate extended-release tablets 4 mg (N=42), fesoterodine fumarate extended-release tablets 8 mg (N=42), or active comparator (N=40) orally once daily. The majority of patients were Caucasian (52%) or Asian (44%) with a mean age of 11 years (range 6 years to 17 years) and a mean weight of 42.8 kg (range 25.1 to 96.0 kg).
Primary Endpoint
The primary efficacy endpoint was the mean change from baseline in maximum cystometric bladder capacity (MCBC) at Week 12.
Treatment with fesoterodine fumarate extended-release tablets 4 mg or 8 mg daily resulted in improvements from baseline to Week 12 in the primary efficacy endpoint, MCBC, for pediatric patients, with numerically higher changes from baseline for fesoterodine fumarate extended-release tablets 8 mg daily than for fesoterodine fumarate extended-release tablets 4 mg daily.
Results for the primary endpoint MCBC are reported in Table 11.
Table 11: Mean Baseline and Change From Baseline to Week 12 for Maximum Cystometric Bladder Capacity (mL) in Pediatric NDO Patients Receiving Fesoterodine Fumarate Extended-Release Tablets 4 mg or Fesoterodine Fumarate Extended-Release Tablets 8 mg and Weighing More Than 25 kg in Study 3 CI = confidence interval
Baseline is defined as the last available measurement prior to the start of treatment.
N is the number of patients who took at least one dose and provided a valid value for MCBC at baseline.
† Least squares mean change and 95% CI are based on an analysis of covariance model with terms for treatment group, baseline maximum cystometric bladder capacity and baseline weight. Last observation carried forward/baseline observation carried forward was used for imputing missing values at Week 12.
Fesoterodine Fumarate Extended-Release Tablets 4 mg
Fesoterodine Fumarate Extended-Release Tablets 8 mg
N
41
41
Baseline
195.1
173.3
Change from baseline (95% CI)†
58.1
(28.8, 87.4)
83.4
(54.2, 112.5)
Secondary Endpoints
Results for other urodynamic secondary efficacy endpoints and selected secondary efficacy endpoints derived from patient urinary diaries are reported in Tables 12 and 13, respectively.
Table 12: Summary of Baseline and Change From Baseline to Week 12 in Secondary Urodynamic Endpoints in Pediatric NDO Patients Weighing Greater Than 25 kg in Study 3 Fesoterodine Fumarate Extended-Release Tablets
4 mg
Fesoterodine Fumarate Extended-Release Tablets
8 mg
Detrusor pressure at maximum bladder capacity (cmH2O)
N
40
41
Baseline
26.5
27.2
Change from Baseline (95% CI)†
-2.9
(-7.6, 1.9)
-1.6
(-6.3, 3.1)
Number and percentage of patients with IDC at Baseline but not at Week 12 (n (%))
N
41
41
n (%)
9 (22.0)
18 (43.9)
Bladder volume at first IDC (mL)
N
26
36
Baseline
88.6
88.5
Change from Baseline (95% CI)†
30.5
(2.4, 58.6)
26.1
(2.2, 49.9)
Bladder compliance (mL/cmH20)
N
40
40
Baseline
13.8
10.1
Change from Baseline (95% CI)†
6.4
(-0.5, 13.3)
5.4
(-1.5, 12.3)
CI = confidence interval; IDC = involuntary detrusor contractions
Baseline is defined as the last available measurement prior to the start of treatment.
N is the number of patients who took at least one dose and provided valid endpoint data at baseline.† Least squares mean change and 95% CI are based on an analysis of covariance model with terms for treatment group, baseline (for the endpoint being analyzed) and baseline weight. Last observation carried forward/baseline observation carried forward was used for imputing missing values at Week 12.
Table 13: Mean Baseline and Change From Baseline to Week 12 in Selected Secondary Bladder-Diary Endpoints in Pediatric NDO Patients Weighing Greater Than 25 kg in Study 3 CI = confidence interval
Baseline is defined as the last available measurement prior to the start of treatment.
N is the number of patients who took at least one dose and provided valid endpoint data at baseline.
§ Only patients with >0 incontinence episodes at baseline are included.
† Least squares mean change and 95% CI are based on an analysis of covariance model with terms for treatment group, baseline (for the endpoint being analyzed) and baseline weight. Last observation carried forward/baseline observation carried forward was used for imputing missing values at Week 12.
Fesoterodine Fumarate Extended-Release Tablets
4 mg
Fesoterodine Fumarate Extended-Release Tablets
8 mg
Number of incontinence episodes per 24 hours§
N
33
33
Baseline
2.8
2.7
Change from Baseline (95% CI)†
-0.5
(-0.9, 0.0)
-0.9
(-1.4, -0.4)
Maximum catheterized urine volume per 24 hours (mL)
N
36
32
Baseline
222.5
164.7
Change from Baseline (95% CI)†
31.6
(-9.3, 72.6)
51.0
(8.1, 93.8)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Fesoterodine fumarate extended-release tablets 4 mg are light blue, oval, biconvex, film coated tablets debossed with “K46” on one side and plain on other side. They are supplied as follows:
Bottles of 30 NDC 65862-766-30
Fesoterodine fumarate extended-release tablets 8 mg are blue, oval, biconvex, film coated tablets debossed with “K47” on one side and plain on other side. They are supplied as follows:
Bottles of 30 NDC 65862-767-30
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from moisture. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-Approved Patient Labeling (Patient Information).
Angioedema
Inform patients and/or their caregivers that fesoterodine fumarate extended-release tablets may cause angioedema, which could result in life-threatening airway obstruction. Advise patients and/or their caregivers to promptly discontinue fesoterodine fumarate extended-release tablets and seek immediate medical attention if they experience edema of the lips, tongue or laryngopharynx, or difficulty breathing.
Antimuscarinic Effects
Inform patients that fesoterodine fumarate extended-release tablets, like other antimuscarinic agents, may produce clinically significant adverse effects related to antimuscarinic pharmacological activity including constipation and urinary retention. Fesoterodine fumarate extended-release tablets, like other antimuscarinics, may be associated with blurred vision, therefore, patients should be advised to exercise caution in decisions to engage in potentially dangerous activities until the drug’s effects on the patient have been determined. Heat prostration (due to decreased sweating) can occur when fesoterodine fumarate extended-release tablets, like other antimuscarinic drugs, is used in a hot environment.
Alcohol
Patients should also be informed that alcohol may enhance the drowsiness caused by fesoterodine fumarate extended-release tablets, like other anticholinergic agents. -
PATIENT PACKAGE INSERT
Patient Information
Fesoterodine Fumarate (fes″ oh ter′ oh deen fue′ ma rate)
Extended-Release Tablets, for oral use
Read the Patient Information that comes with fesoterodine fumarate extended-release tablets before you start taking them and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What are fesoterodine fumarate extended-release tablets?
Fesoterodine fumarate extended-release tablets are a prescription medicine used:
- in adults to treat symptoms of a condition called overactive bladder (OAB), including urge urinary incontinence (leaking or wetting accidents due to a strong need to urinate), urinary urgency (having a strong need to urinate right away), or urinary frequency (having to urinate too often).
- in children 6 years of age and older with a body weight greater than 55 pounds (25 kg) to treat neurogenic detrusor overactivity (NDO). Fesoterodine fumarate extended-release tablets are used to increase the amount of urine your bladder can hold and reduce urine leakage.
Who should not take fesoterodine fumarate extended-release tablets?
Do not take fesoterodine fumarate extended-release tablets if you:
- are allergic to fesoterodine fumarate extended-release tablets or any of its ingredients. See the end of this leaflet for a complete list of ingredients.
- are allergic to tolterodine tartrate tablets or tolterodine tartrate extended-release capsules.
- are not able to empty your bladder (urinary retention).
- have delayed or slow emptying of your stomach (gastric retention).
- have an eye problem called uncontrolled narrow-angle glaucoma.
Before you take fesoterodine fumarate extended-release tablets, tell your healthcare provider about all your medical conditions, including if you:
- have problems emptying your bladder or you have a weak urine stream.
- have any stomach or intestinal problems, or problems with constipation.
- are receiving treatment for an eye problem called narrow-angle glaucoma.
- have a condition called Myasthenia Gravis.
- have kidney problems.
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if fesoterodine fumarate extended-release tablets will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if fesoterodine fumarate passes into your breast milk. You should talk to your healthcare provider about the best way to feed your baby while taking fesoterodine fumarate extended-release tablets.
Know all the medicines you take. Keep a list of them with you to show your healthcare provider and pharmacist each time you get a new medicine.
How should I take fesoterodine fumarate extended-release tablets?
- Take fesoterodine fumarate extended-release tablets exactly as your healthcare provider tells you to take them.
- Your healthcare provider may lower your dose of fesoterodine fumarate extended-release tablets if you are an adult with severe kidney problems.
- Your healthcare provider may lower or stop your dose of fesoterodine fumarate extended-release tablets if you are a child 6 years of age and older with a body weight greater than 77 pounds (35 kg) and have severe kidney problems or are taking certain medicines.
- Take fesoterodine fumarate extended-release tablets with liquid and swallow the tablet whole. Do not chew, divide, or crush the tablet.
- Take fesoterodine fumarate extended-release tablets with or without food.
- If you miss a dose of fesoterodine fumarate extended-release tablets, begin taking fesoterodine fumarate extended-release tablets again the next day. Do not take 2 doses of fesoterodine fumarate extended-release tablets in the same day.
- If you take too much fesoterodine fumarate, call your healthcare provider or go to an emergency department right away.
What should I avoid while taking fesoterodine fumarate extended-release tablets?
- Fesoterodine fumarate extended-release tablets can cause blurred vision, dizziness, and drowsiness. Do not drive, operate machinery, or do other dangerous activities until you know how fesoterodine fumarate extended-release tablets affect you.
- Use caution in hot environments. Decreased sweating and severe heat illness can happen when medicines such as fesoterodine fumarate extended-release tablets are used in a hot environment.
- Drinking alcohol while taking medicines such as fesoterodine fumarate extended-release tablets may cause increased drowsiness.
What are the possible side effects of fesoterodine fumarate extended-release tablets?
Fesoterodine fumarate extended-release tablets may cause serious side effects, including:
- serious allergic reactions. Symptoms of a serious allergic reaction may include swelling of the face, lips, throat, or tongue. If you have any of these symptoms, you should stop taking fesoterodine fumarate extended-release tablets and get emergency medical help right away.
- inability to empty bladder (urinary retention). Fesoterodine fumarate extended-release tablets may increase your chances of not being able to empty your bladder if you have bladder outlet obstruction. Tell your healthcare provider right away if you are unable to empty your bladder.
- central nervous system (CNS) effects. Talk to your healthcare provider right away if you get any of these side effects: headache, dizziness, and drowsiness.
- worsening of Myasthenia Gravis symptoms.
- dry mouth
- constipation
- diarrhea
- dry mouth
- stomach pain
- weight gain
- urinary tract infection
- constipation
- nausea
- headache
How should I store fesoterodine fumarate extended-release tablets?
- Store fesoterodine fumarate extended-release tablets at room temperature between 68° to 77°F (20° to 25°C).
- Protect the medicine from moisture by keeping the bottle closed tightly.
- Keep fesoterodine fumarate extended-release tablets and all medicines out of the reach of children.
General information about the safe and effective use of fesoterodine fumarate extended-release tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use fesoterodine fumarate extended-release tablets for a condition for which it was not prescribed. Do not give fesoterodine fumarate extended-release tablets to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about fesoterodine fumarate extended-release tablets that is written for health professionals.
What are the ingredients in fesoterodine fumarate extended-release tablets?
Active ingredient: fesoterodine fumarate.
Inactive ingredients: FD&C Blue #2/indigo carmine aluminum lake, glyceryl dibehenate, hypromellose, lactose monohydrate, lecithin (soya), microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and xylitol.
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
For more information, call Aurobindo Pharma USA, Inc. at 1-866-850-2876.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 12/2024
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 4 mg (30 Tablets Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 8 mg (30 Tablets Bottle)
-
INGREDIENTS AND APPEARANCE
FESOTERODINE FUMARATE
fesoterodine fumarate tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65862-766 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FESOTERODINE FUMARATE (UNII: EOS72165S7) (FESOTERODINE - UNII:621G617227) FESOTERODINE FUMARATE 4 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 2 (UNII: L06K8R7DQK) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) HYPROMELLOSE 2208 (100000 MPA.S) (UNII: VM7F0B23ZI) HYPROMELLOSE 2208 (4000 MPA.S) (UNII: 39J80LT57T) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MICROCRYSTALLINE CELLULOSE 112 (UNII: X7XJ6RM9Q2) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color BLUE (ligth blue) Score no score Shape OVAL (biconvex) Size 13mm Flavor Imprint Code K46 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65862-766-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205007 06/17/2022 FESOTERODINE FUMARATE
fesoterodine fumarate tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65862-767 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FESOTERODINE FUMARATE (UNII: EOS72165S7) (FESOTERODINE - UNII:621G617227) FESOTERODINE FUMARATE 8 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 2 (UNII: L06K8R7DQK) GLYCERYL DIBEHENATE (UNII: R8WTH25YS2) HYPROMELLOSE 2208 (100000 MPA.S) (UNII: VM7F0B23ZI) HYPROMELLOSE 2208 (4000 MPA.S) (UNII: 39J80LT57T) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MICROCRYSTALLINE CELLULOSE 112 (UNII: X7XJ6RM9Q2) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color BLUE Score no score Shape OVAL (biconvex) Size 13mm Flavor Imprint Code K47 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65862-767-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/17/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA205007 06/17/2022 Labeler - Aurobindo Pharma Limited (650082092) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650381903 ANALYSIS(65862-766, 65862-767) , MANUFACTURE(65862-766, 65862-767)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.