Careline by COSMOPHARM LTD. / Emilia Cosmetics Ltd Careline
Careline by
Drug Labeling and Warnings
Careline by is a Otc medication manufactured, distributed, or labeled by COSMOPHARM LTD., Emilia Cosmetics Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CARELINE
ANTIGRAVITY- octocrylene,avobenzone,allantoin cream
COSMOPHARM LTD.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Careline
Purpose
OCTOCRYLENE.............................................................................Sunscreen
BUTYL METHOXYDIBENZOYLMETHANE 3.15%..............................Sunscreen
ALLANTOIN 0.2%..........................................................................Skin Protectant
Uses:
- helps prevent sunburn
- if used as directed with other sun protection measures (See directions) decreases the risk of cancer and early skin aging causes by the sun.
Warnings:
For external use only.
Directions:
For sunscreen use. Apply liberally to face and neck 15 minutes before sun exposure. Apply at least every 2 hours
Sun Protection Measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit your time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeve shirts, pants, hats and sunglasses.
- Reapply at least every 2 hours.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply immediately after towel drying.
Inactive ingredients:
Purified Water, Cyclopentasiloxane, Dimethicone Crosspolymer, Glycerin, Isohexadecane, Behenyl Alcohol, Glyceryl Stearate, Glyceryl Stearate Citrate, Disodium Ethylene Dicocamide PEG-15 Disulfate, Butylene Glycol, Niacinamide, Polyacrylamide, C13-14 Isoparaffin, Laureth-7, Glycerin, Dextran, Trifluoroacetyl Tripeptide-2, Propanediol, Ornithine, Phospholipids, Glycolipids, Polyethylene, Stearyl Alcohol, Phenoxyethanol, Ethylhexylglycerin, Parfum, Tocopheryl Acetate (Vitamin E), Xanthan Gum,Panthenol (Pro-Vitamin B5), Disodium EDTA, Bisabolol, Hyaluronic Acid, Alpha Isomethyl Ionone, Benzyl Salicylate, Butylphenyl Methylpropional, Citronellol, Hexyl Cinnamal, Hydroxyisohexyl-3-Cyclohexene Carboxaldehyde, Limonene, CI 14700.
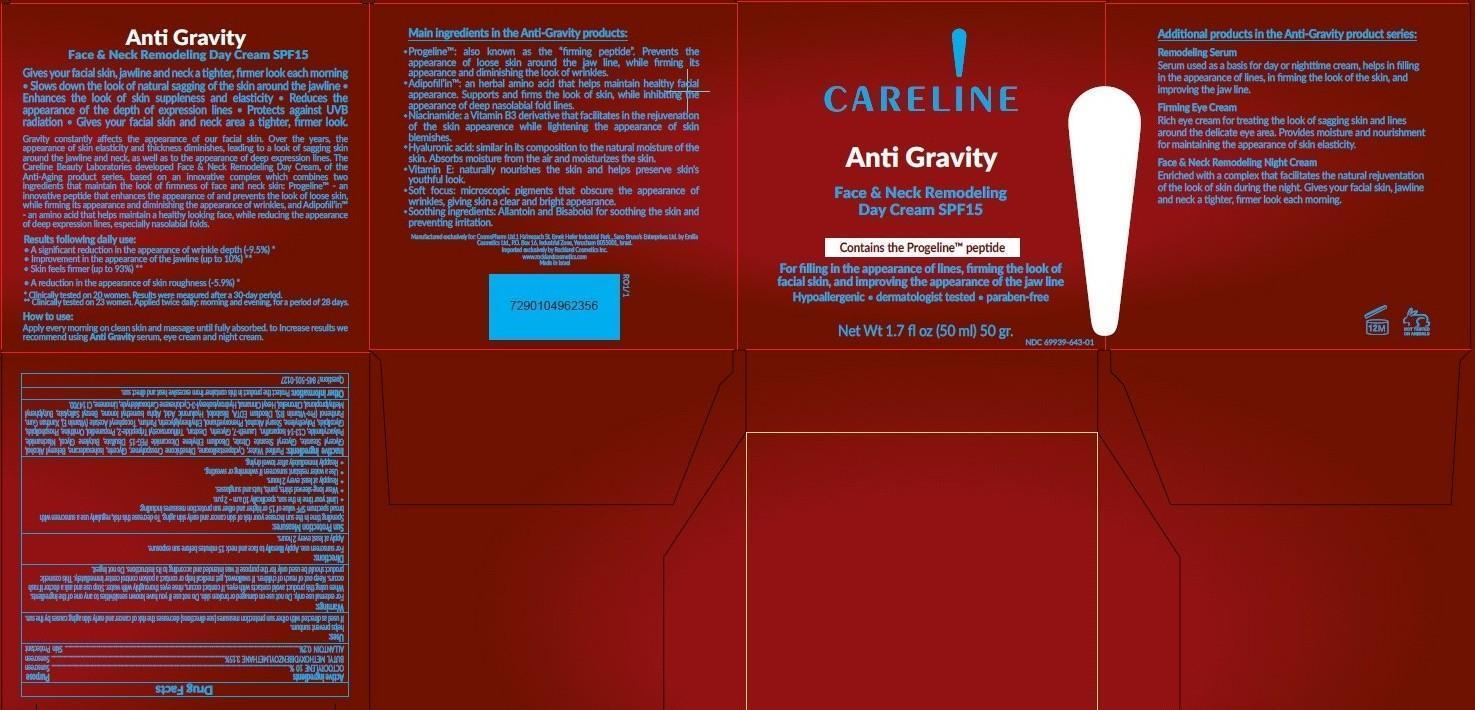
Principal Display Panel
Careline
Anti Gravity
Face and Neck Remodeling
Day Cream
SPF 15
Contains the Progeline peptide
For filling in the appearance of lines, firming the look of facial skin, and improving the appearance of the jaw line
Hypoallergenic dermatologist tested paraben free
Net Wt. 1.7 fl oz (50 ml) 50 gr.
NDC: 69939-643-01
| CARELINE
ANTIGRAVITY
octocrylene,avobenzone,allantoin cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - COSMOPHARM LTD. (600395487) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Emilia Cosmetics Ltd | 600076624 | manufacture(69939-643) | |