ACNE BLU SALICYLIC ACID PADS- salicylic acid disc
Acne Blu Salicylic Acid Pads by
Drug Labeling and Warnings
Acne Blu Salicylic Acid Pads by is a Otc medication manufactured, distributed, or labeled by INK Pharmaceuticals, Inc., Swiss-American CDMO, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
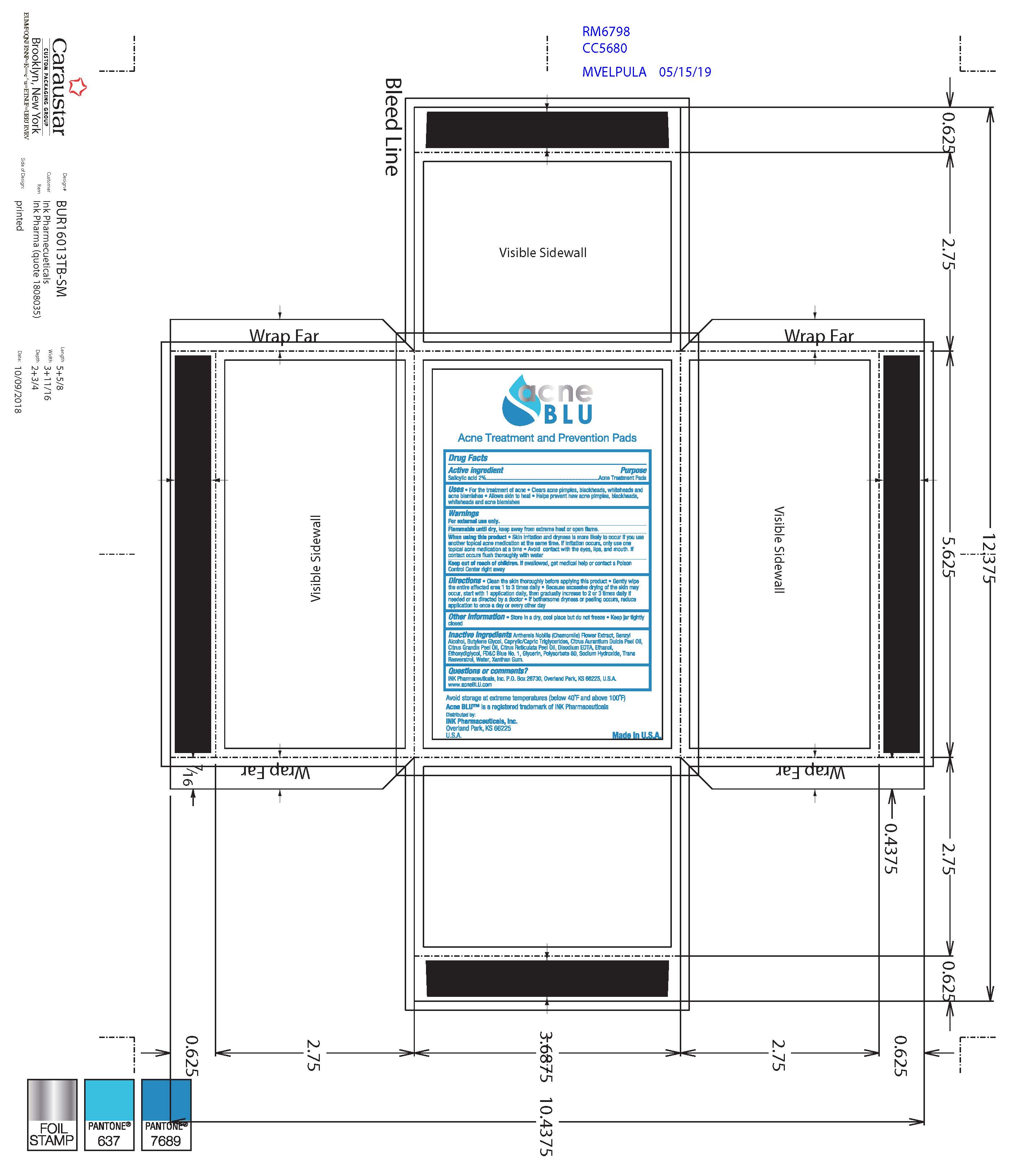
Warnings
For external use only
Flammable until dry, keep away from extremem heat or open flame
When using this product skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time. Avoid contact with the eyes, lips and mouth. If contact occurs flush throughly with water.
If swallowed, get medical help or contact a Poison Control Center right away.
- Keep out of reach of children
- Active Ingredients
- Uses
- Uses
-
Directions
Clean the skin thoroughly before applying this product. Gently wipe the entire affected area 1 to 3 times daily. Because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Other Information
-
Incative Ingredients
Anthemis Nobilis (Chamomile) Flower Extract, Benzyl Alcohol, Butylene Glycol, Caprylic/Capric Triglycerides, Citrus Aurantium Dulcis Peel Oil, Citrus Grandis Peel Oil, Citrus Reticulata Peel Oil, Disodium EDTA, EThanol, Ethoxydiglycol, FD&C Blue #1, Glycerin, Polysorbate 80, Sodium Hydroxide, Trans Resveratrol, Water, Xanthan Gum
- Questions or comments?
- Bottom of Box
-
INGREDIENTS AND APPEARANCE
ACNE BLU SALICYLIC ACID PADS
salicylic acid discProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70547-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 g in 1000 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) ALCOHOL (UNII: 3K9958V90M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) BENZYL ALCOHOL (UNII: LKG8494WBH) POLYSORBATE 80 (UNII: 6OZP39ZG8H) RESVERATROL (UNII: Q369O8926L) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ORANGE PEEL (UNII: TI9T76XD44) CITRUS MAXIMA FRUIT RIND OIL (UNII: 8U3877WD44) MANDARIN OIL (UNII: NJO720F72R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70547-200-90 90 in 1 JAR 01/06/2020 1 113 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 01/06/2020 Labeler - INK Pharmaceuticals, Inc. (086964278) Registrant - Swiss-American CDMO, LLC (080170933) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(70547-200)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.