PHENYLEPHRINE HYDROCHLORIDE solution/ drops
Phenylephrine Hydrochloride by
Drug Labeling and Warnings
Phenylephrine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Bausch & Lomb Americas Inc., Jubilant HollisterStier General Partnership. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PHENYLEPHRINE HYDROCHLORIDE OPHTHALMIC SOLUTION, USP 2.5% and 10% safely and effectively. See full prescribing information for PHENYLEPHRINE HYDROCHLORIDE OPHTHALMIC SOLUTION, USP 2.5% and 10%.
PHENYLEPHRINE HYDROCHLORIDE OPHTHALMIC SOLUTION, USP 2.5% and 10%, for topical ophthalmic use
Initial U.S. Approval: 1939INDICATIONS AND USAGE
Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% and 10% is an alpha-1 adrenergic receptor agonist indicated to dilate the pupil (1).
DOSAGE AND ADMINISTRATION
- For patients 1 year of age and older, apply one drop of Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% or 10% at 3 to 5 minute intervals up to a maximum of 3 drops per eye (2.1).
- In pediatric patients less than 1 year of age, one drop of Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% should be instilled at 3 to 5 minute intervals up to a maximum of 3 drops per eye (2.2).
DOSAGE FORMS AND STRENGTHS
2.5% and 10% sterile ophthalmic solutions (3).
CONTRAINDICATIONS
- Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% is contraindicated in patients with hypertension, or thyrotoxicosis. Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% should be used in these patients (4.1).
- Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% is contraindicated in pediatric patients less than 1 year of age due to the increased risk of systemic toxicity. Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% should be used in these patients (4.2).
WARNINGS AND PRECAUTIONS
- Not for injection. Phenylephrine Hydrochloride Ophthalmic Solution, USP is indicated for topical ophthalmic use (5.1).
- Reports of serious cardiovascular reactions, some fatal, with Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% solution. Monitor blood pressure in patients with cardiovascular disease (5.2).
- Significant elevations in blood pressure have been reported. Caution in pediatric patients less than 5 years of age, and in patients with elevated blood pressure (5.3).
- Rebound miosis has been reported one day after instillation (5.4).
ADVERSE REACTIONS
- Ocular adverse reactions include eye pain and stinging on instillation, temporary blurred vision, and photophobia (6.1).
-
Cardiovascular adverse reactions include increase in blood pressure, syncope, myocardial infarction, tachycardia, arrhythmia and subarachnoid hemorrhage (6.2).
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Recommendations
2.2 Pediatric Patients Less Than 1 Year of Age
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Cardiac and Endocrine Disease
4.2 Pediatric Patients Less Than 1 Year of Age
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use
5.2 Cardiovascular Reactions
5.3 Elevation in Blood Pressure
5.4 Rebound Miosis
6 ADVERSE REACTIONS
6.1 Ocular Adverse Reactions
6.2 Systemic Adverse Reactions
7 DRUG INTERACTIONS
7.1 Agents That May Exaggerate Pressor Responses
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Recommendations
For patients 1 year of age or greater, apply one drop of either Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% or 10% every 3 to 5 minutes to the conjunctival fornix as required up to a maximum of 3 drops. If necessary, this dose may be repeated.
In order to obtain a greater degree of mydriasis, Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% may be needed.
-
3 DOSAGE FORMS AND STRENGTHS
Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% is a clear, colorless, sterile topical ophthalmic solution containing phenylephrine hydrochloride 2.5%.
Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% is a clear, colorless, sterile topical ophthalmic solution containing phenylephrine hydrochloride 10%.
-
4 CONTRAINDICATIONS
4.1 Cardiac and Endocrine Disease
Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% is contraindicated in patients with hypertension, or thyrotoxicosis. Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% should be used in these patients.
4.2 Pediatric Patients Less Than 1 Year of Age
Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% is contraindicated in pediatric patients less than 1 year of age due to the increased risk of systemic toxicity. Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% should be used in these patients [See Dosage and Administration(2.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use
Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% and 10% is not indicated for injection.
5.2 Cardiovascular Reactions
There have been reports of serious cardiovascular reactions, including ventricular arrhythmias and myocardial infarctions, in patients using phenylephrine 10%. These episodes, some fatal, have usually occurred in patients with pre-existing cardiovascular diseases. Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% should be used in these patients.
5.3 Elevation in Blood Pressure
A significant elevation in blood pressure is not common but has been reported following conjunctival instillation of recommended doses of phenylephrine 10%. The risk is less with phenylephrine 2.5%. Caution should be exercised with the use of phenylephrine 10% in pediatric patients less than 5 years of age and patients with hyperthyroidism or cardiovascular disease. The post-treatment blood pressure of patients with cardiac and endocrine diseases and any patients who develop symptoms should be carefully monitored.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Cardiac Disease [See Warnings and Precautions (5.2)].
- Elevation in Blood Pressure [See Warnings and Precautions (5.3)].
The following adverse reactions have been identified following use of phenylephrine hydrochloride ophthalmic solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
6.1 Ocular Adverse Reactions
Eye pain and stinging on instillation, temporary blurred vision and photophobia, and conjunctival sensitization may occur.
6.2 Systemic Adverse Reactions
A marked increase in blood pressure has been reported particularly, but not limited to, low weight premature neonates, infants and hypertensive patients.
Cardiovascular reactions which have occurred primarily in hypertensive patients following topical ocular use of phenylephrine hydrochloride ophthalmic solution 10% include marked increase in blood pressure, syncope, myocardial infarction, tachycardia, arrhythmia and subarachnoid hemorrhage [See Warnings and Precautions (5.2 and5.3)].
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted with topical phenylephrine. It is also not known whether phenylephrine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% and 10% should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human breast milk. Because many drugs are excreted in human milk, caution should be exercised when Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% and 10% is administered to a nursing woman.
8.4 Pediatric Use
Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% is contraindicated in pediatric patients less than 1 year of age. [See Contraindications (4.2)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
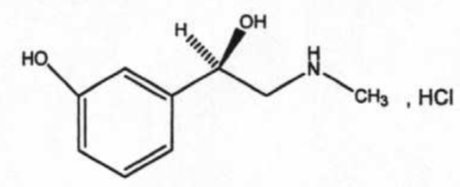
Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% and 10% is a sterile, clear, colorless, topical mydriatic agent for ophthalmic use. The chemical name is (R)-3-hydroxy-α-[(methylamino)methyl]benzenemethanol hydrochloride. Phenylephrine hydrochloride is represented by the following structural formula:
Phenylephrine hydrochloride has a molecular weight of 203.67 and an empirical formula of C9H13NO2-HCl.
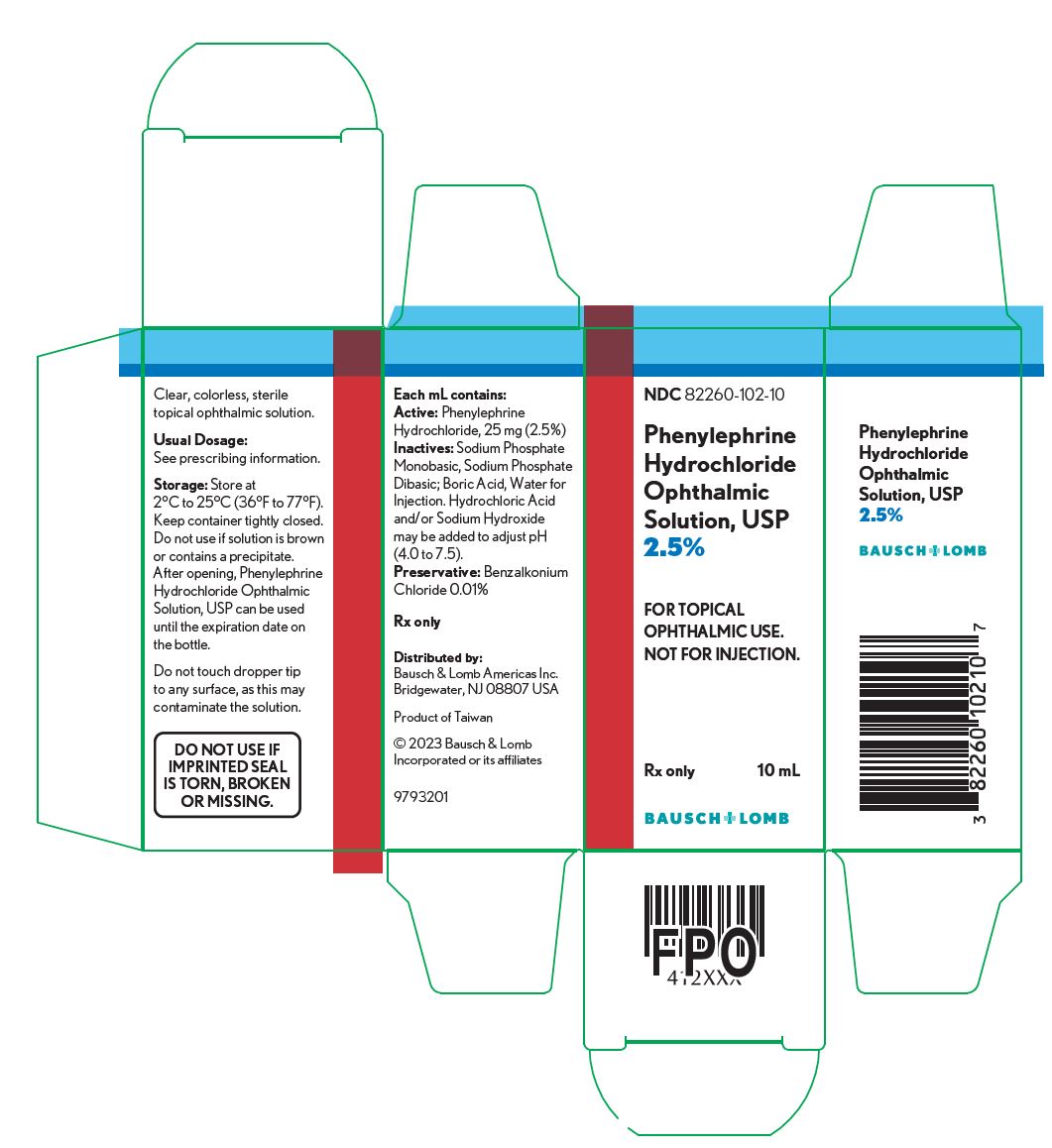
Each mL of Phenylephrine Hydrochloride Ophthalmic Solution, 2.5% contains: ACTIVE: phenylephrine hydrochloride 25 mg (2.5%); INACTIVES: sodium phosphate monobasic, sodium phosphate dibasic; boric acid, water for injection. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH (4.0-7.5). The solution has a tonicity of 500 mOsm/kg; PRESERVATIVE: benzalkonium chloride 0.01%.
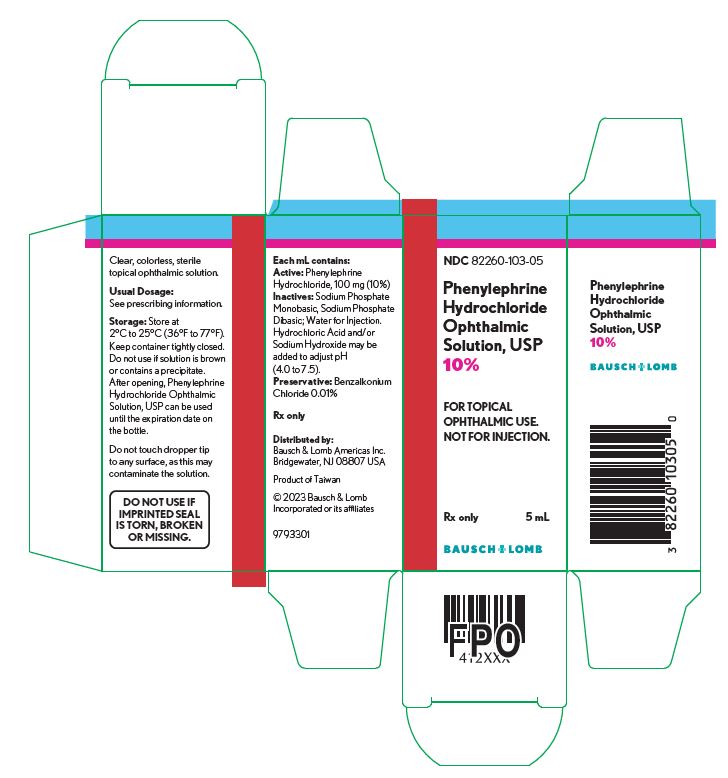
Each mL of Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% contains: ACTIVE: phenylephrine hydrochloride 100 mg (10%); INACTIVES: sodium phosphate monobasic, sodium phosphate dibasic; water for injection. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH (4.0-7.5). The solution has a tonicity of 1000 mOsm/kg; PRESERVATIVE: benzalkonium chloride 0.01%.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Phenylephrine is an alpha-1 adrenergic receptor agonist used for dilation of the pupil due to its vasoconstrictor and mydriatic action. Phenylephrine possesses predominantly α-adrenergic effects. In the eye, phenylephrine acts locally as a potent vasoconstrictor and mydriatic, by constricting ophthalmic blood vessels and the radial muscle of the iris.
12.2 Pharmacodynamics
Maximal mydriasis occurs in 20-90 minutes with recovery after 3 to 8 hours.
Systemic absorption of sufficient quantities of phenylephrine may lead to systemic α-adrenergic effects, such as rise in blood pressure which may be accompanied by a reflex atropine-sensitive bradycardia.
-
14 CLINICAL STUDIES
Pupillary dilation following topical administration of phenylephrine hydrochloride ophthalmic solution has been demonstrated in controlled clinical studies in adults and pediatric patients with different levels of iris pigmentation. Pupil movement is generally seen within 15 minutes, maximal mydriasis between 20 to 90 minutes and recovery after 3 to 8 hours. Darker irides tend to dilate slower than lighter irides.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% is supplied as a sterile, aqueous, topical ophthalmic solution with a fill volume of 10 mL in a 10 mL opaque, white low density polyethylene (LDPE) bottle with a linear low density polyethylene (LLDPE) dropper tip and red cap (NDC: 82260-102-10). Phenylephrine HCl Ophthalmic Solution, USP 10% is supplied as a sterile, aqueous, topical ophthalmic solution supplied with a fill volume of 5 mL in a 10 mL opaque, white LDPE bottle with an LLDPE dropper tip and red cap (NDC: 82260-103-05).
-
17 PATIENT COUNSELING INFORMATION
Advise patients not to touch the dropper tip to any surface as this may contaminate the solution. Inform patients they may experience sensitivity to light and should protect their eyes in bright illumination while their pupils are dilated.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
© 2023 Bausch & Lomb Incorporated or its affiliates
9793400
-
PRINCIPAL DISPLAY PANEL
NDC: 82260-102-10
Phenylephrine
Hydrochloride
Ophthalmic
Solution, USP
2.5%
FOR TOPICAL
OPHTHALMIC USE.
NOT FOR INJECTION.
Rx only
10 mL
9793201
-
Package/Label Display Panel
NDC: 82260-103-05
Phenylephrine
Hydrochloride
Ophthalmic
Solution, USP
10%FOR TOPICAL
OPHTHALMIC USE.
NOT FOR INJECTION.Rx Only
5 mL
9793301
-
INGREDIENTS AND APPEARANCE
PHENYLEPHRINE HYDROCHLORIDE
phenylephrine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 82260-102 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) BORIC ACID (UNII: R57ZHV85D4) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82260-102-15 1 in 1 CARTON 03/31/2023 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 2 NDC: 82260-102-10 1 in 1 CARTON 03/31/2023 2 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA203510 03/31/2023 PHENYLEPHRINE HYDROCHLORIDE
phenylephrine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 82260-103 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) BORIC ACID (UNII: R57ZHV85D4) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82260-103-05 1 in 1 CARTON 03/31/2023 1 5 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA203510 03/31/2023 Labeler - Bausch & Lomb Americas Inc. (118287629) Establishment Name Address ID/FEI Business Operations Jubilant HollisterStier General Partnership 246762764 MANUFACTURE(82260-102, 82260-103)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.