GLYCOPHOS- sodium glycolate injection, solution
Glycophos by
Drug Labeling and Warnings
Glycophos by is a Prescription medication manufactured, distributed, or labeled by Fresenius Kabi USA, LLC, Fresenius Kabi Norge AS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HEALTH CARE PROVIDER LETTER
IMPORTANT PRESCRIBING INFORMATION
October 30, 2017Subject: Temporary importation of Glycophos to address drug shortage issues
Dear Healthcare Professional,
Due to the critical shortage of phosphate injection in the U.S. market, Fresenius Kabi USA, LLC (Fresenius Kabi USA) is coordinating with the U.S. Food and Drug Administration (FDA) to provide an alternative treatment option during this critical shortage period. Fresenius Kabi USA has initiated temporary importation of a GlycophosTM 20 mL Injection Single Dose Plastic Vial into the U.S. market. This product is marketed in Europe, and is manufactured in the Fresenius Kabi Norway plant.At this time, no other entity except Fresenius Kabi USA is authorized by the FDA to import or distribute GlycophosTM 20 mL Injection Single Dose Plastic Vial in the U.S. FDA has not approved Fresenius Kabi's GlycophosTM in the United States.
Effective immediately, and during this temporary period, Fresenius Kabi USA will offer the following presentation of phosphate injection:
Glycophos 20 mL
Sterile Concentrate
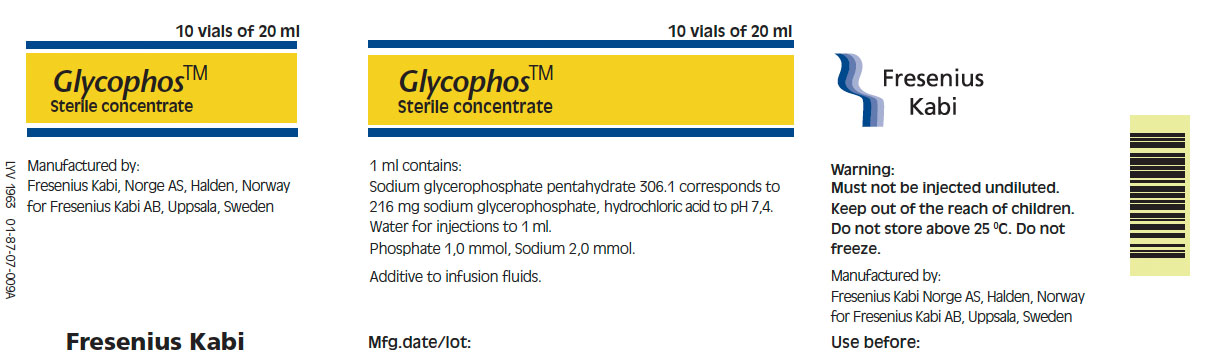
Single Dose Plastic VialChemical Name Sodium Glycerophosphate Phosphate Concentration 1 mMol per mL. Type of Phosphate Organic Sodium 2 mEq per mL. Fill Volume 20 mL. Description Single Dose Plastic Vial Manufacturer Fresenius Kabi Norge A/S The vial and carton labels will display the text used when marketing the product in English speaking countries.
It is important to note that there are some key differences in the formulation and labeling
between the current U.S. marketed phosphate injection products and Glycophos that you need to be aware of:

Refer to the Glycophos package insert for full prescribing information
This communication and product information is available on the Fresenius Kabi USA web site http://products.fresenius-kabi.us/product-323.html as well as on the FDA Drug Shortage web site. http://www.fda.gov/Drugs/DrugSafety/DrugShortages/default.htm.To report adverse events or quality problems experienced with the use of this product, call Fresenius Kabi USA Vigilance or Medical Affairs at 1-800-551-7176, Monday – Friday, between the hours of 8 a.m. and 5 p.m. (CST), or e-mail adverse.events.USA@fresenius-kabi.com or productcomplaint.USA@fresenius-kabi.com.
Fresenius Kabi USA CONTACT NUMBERS: Please use the following contact numbers as appropriate:
Reason To Call Department Number ADE Reporting Vigilance Department 1-800-551-7176 Clinical/Technical
Info. Or Product
ComplaintMedical Affairs Department Product Availability & Ordering Customer Service Department 1-888-386-1300
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/medwatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
Sincerely,
Melanie Power-Burns
Vice President, Quality and Compliance
Key Differences between U.S. Marketed Phosphate Injection Products and GlycophosCurrent U.S. Marketed
Inorganic Phosphate Injection, USPGlycophos What does this mean to you,
as a Healthcare Professional?Indications and contraindications:
see package insertIndications and contraindications:
see package insertGlycophos is indicated in adult patients and
infants as a supplement in intravenous nutrition
to meet the requirements of phosphate.
Glycophos is contraindicated in patients
in a state of dehydration or with hypernatremia,
hyperphosphatemia, severe renal insufficiency or shock.
Sodium Phosphates and
Potassium Phosphates
contain 3 mMol of
phosphate per mL.Glycophos contains 1 mMol
of phosphate per mL.
Glycophos contains 20 mLs in each plastic vial
for a total concentration of 20 mMols of phosphate per vial.
Glycophos must be diluted before administration.
Sodium Phosphates and
Potassium Phosphates are
INORGANIC PHOSPHATE.Glycophos is an
ORGANIC PHOSPHATE.
Organic phosphates tend to be more calcium compatible1.
This means:
- At higher concentrations, solutions of calcium and phosphate may
exist together without precipitating into an insoluble salt complex. - In high pH solutions (admixtures above pH 6.0), organic
phosphate is less likely to precipitate.
Barcode on container labelNo unit of use barcode Any barcodes on Glycophos product will not be
appropriately recognized by scanning systems
used in the United States and should NOT be used.
Institutions should manually input the product into their
systems and to confirm that barcode systems do not
provide incorrect information when the product is scanned.For questions regarding Glycophos in the United States, please contact
Fresenius Kabi USA Medical Affairs at 1-800-551-7176 Option 4,
Monday – Friday, between the hours of 8 a.m. and 5 p.m. (CST), or e-mail nutrition.medinfo.USA@fresenius-kabi.com.
1. Data on file.
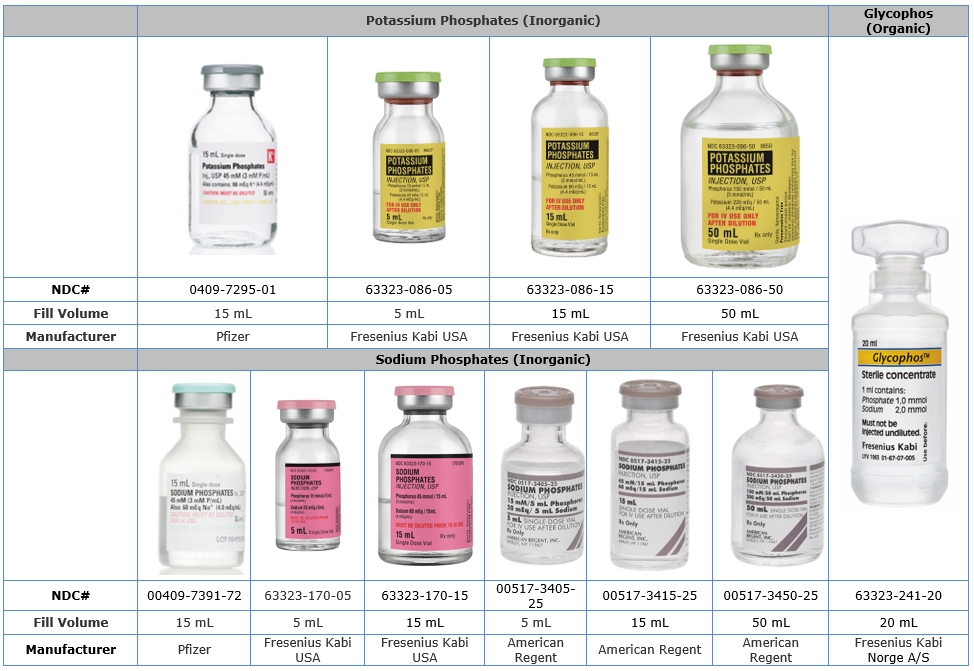
Comparison Table of U.S. Phosphate Injection Products to Glycophos
Product Name Potassium Phosphates Sodium Phosphates Glycophos Chemical Name Potassium Phosphate Sodium Phosphate Sodium Glycerophosphate Phosphate Concentration 3 mMol per mL 3 mMol per mL 1 mMol per mL Type of Phosphate Inorganic Inorganic Organic Sodium Does not contain 4 mEq per mL 2 mEq per mL Potassium 4.4 mEq per mL Does not contain Does not contain Fill Volume 5 mL, 15 mL, 50 mL 5 mL, 15 mL, 50 mL 20 mL Description Single Dose Vial Single Dose Vial Single Dose Plastic Vial Companies Fresenius Kabi USA, Pfizer American Regent,
Fresenius Kabi USA, PfizerFresenius Kabi Norge A/S
Phosphate Label Product Comparison Table
-
MEDICATION GUIDE
1. QUALITATIVE AND QUANTITATIVE COMPOSITION
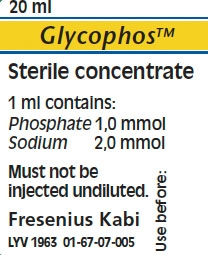
1 ml of Glycophos contains
Active ingredient Quantity
Sodium glycerophosphate pentahydrate 306.1 mg*
*Corresponds to 216 mg sodium glycerophosphate
The active ingredient in 1 ml of Glycophos correspond to
Phosphate 1 mmol
Sodium 2 mmol
For excipients, see 5.1
PRODUCT PROPERTIES
Osmolality: 2760 mosm/kg water
pH: 7.4
2. PHARMACEUTICAL FORM
Concentrate for solution for infusion.
3. CLINICAL PARTICULARS
3.1 Therapeutic indications
Glycophos is indicated in adult patients and infants as a supplement in intravenous nutrition to meet the requirements of phosphate.
3.2 Posology and method of administration
Glycophos must not be given undiluted.
Adults:
The recommended dosage is individual. The recommended daily dosage of phosphate during intravenous nutrition would normally be 10-20 mmol. This can be met by using 10-20 ml of Glycophos added to the infusion solution or to the admixture for which compatibility has been proved.
Infants:
The recommended dosage is individual. The recommended dose for infants and neonates is 1.0-1.5 mmol/Kg body weight/day.
3.3 Contra-indications
Glycophos should not be given to patients in a state of dehydration or with hypernatraemia, hyperphosphataemia, severe renal insufficiency or shock.
3.4 Special warnings and special precautions for use
Glycophos should be used with caution in patients with impaired renal function. The phosphate status of all patients should be monitored regularly.
Glycophos must not be given undiluted.
3.5 Interaction with other medicaments and other forms of interaction
No interactions with other drugs have been observed, but a moderate fall in serum phosphate can be seen during carbohydrate infusions.
3.6 Pregnancy and lactation
Animal reproduction studies or clinical investigations during pregnancy have not been carried out with Glycophos. However, the requirements of phosphate in a pregnant woman are slightly increased compared to non-pregnant women.
No adverse events are to be expected when Glycophos is administered during pregnancy.
3.7 Effects on ability to drive and use machines
No effects on the ability to drive and use machines are to be expected.
3.8 Undesirable effects
No adverse effects related to glycerophosphate have been reported.
3.9 Overdose
No adverse effects of an overdose have been reported. Most patients in need of intravenous nutrition have an increased capacity to handle glycerophosphate. See also 3.3 "Contra-indications".
4. PHARMACOLOGICAL PROPERTIES
4.1 Pharmacodynamic properties
Glycerophosphate is a metabolic intermediate in fat metabolism and any pharmacodynamic effects other than maintaining the normal metabolic pathways are unlikely.
4.2 Pharmacokinetic properties
To become available it is necessary for the phosphate group to be hydrolysed from the glycerophosphate molecule. The hydrolysis occurs maximally at a plasma concentration of >0.7 mmol/l. Assuming that all hydrolysis of glycerophosphate takes place in plasma, about 12-15 mmol of sodium glycerophosphate will be hydrolysed each day in individuals with normal serum alkaline phosphatase.
No pharmacokinetical data is available for infants, however with the recommended dosage hyperphosphataemia is unlikely.
4.3 Preclinical safety data
Preclinical safety studies on Glycophos demonstrated good tolerance.
5. PHARMACEUTICAL PARTICULARS
5.1 List of excipients
Hydrochloric acid
Water for Injections
5.2 Incompatibilities
Glycophos may only be added to or mixed with other medicinal products for which compatibility has been documented. See 5.6.
5.3 Shelf life
3 years
5.4 Special precautions for storage
Do not store above 25°C. Do not freeze.
5.5 Nature and contents of container
Polypropylene vial.
Pack size: 10 x 20 ml
5.6 Instructions for use/handling
Glycophos must not be given undiluted.
Compatibility
Additions should be made aseptically.
Up to 120 ml of Glycophos and 48 mmol of calcium (as CaCl2) can be added to 1000 ml Vamin Glucose, Vamin 9 Electrolyte Free, Vamin 14, Vamin 14 Electrolyte Free, Vamin 18 Electrolyte Free and Vaminolact.
Up to 10 ml of Glycophos and 10 mmol of calcium (as CaCl2) can be added to 1000 ml Glucose 50 mg/ml.
Up to 20 ml of Glycophos and 20 mmol of calcium (as CaCl2) can be added to 1000 ml Glucose 200 mg/ml.
Up to 60 mmol of Glycophos and 24 mmol of calcium (as CaCl2) can be added to 1000 ml Glucose 500 mg/ml.
Infusion time
The infusion time should not be less than 8 hours.
Stability
When additions are made to an infusion solution, the infusion should be completed within 24 hours from preparation to prevent microbiological contamination. The left over contents of opened bottles/vials/ampoules should be discarded and not kept for later use.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GLYCOPHOS
sodium glycolate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63323-241 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM GLYCEROPHOSPHATE ANHYDROUS (UNII: YP1H63LJ2K) (PHOSPHATE ION - UNII:NK08V8K8HR) PHOSPHATE ION 216 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63323-241-20 10 in 1 CARTON 05/13/2013 1 20 mL in 1 VIAL, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 05/13/2013 Labeler - Fresenius Kabi USA, LLC (608775388) Establishment Name Address ID/FEI Business Operations Fresenius Kabi Norge AS 731170932 MANUFACTURE(63323-241)
Trademark Results [Glycophos]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GLYCOPHOS 78408642 2992084 Live/Registered |
Fresenius Kabi AG 2004-04-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.