COLD-EEZE PLUS IMMUNE SUPPORT PLUS ENERGY- zinc gluconate, rosa canina leaf, echinacea purpurea, caffeine, and panax notoginseng root tablet, orally disintegrating
Cold-EEZE by
Drug Labeling and Warnings
Cold-EEZE by is a Homeopathic medication manufactured, distributed, or labeled by ProPhase Labs, Inc., Pharmaloz Manufacturing, Inc., Blispak, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active ingredient (per tablet) Purpose Zincum Gluconicum 1X Cold Remedy Rosa Canina L. (Rose Hip) 1X Promotes Immune support Echinacea Purpurea Extract 2X Promotes Immune support Caffeinum (Natural Caffeine) 1X
P. Quinquefolium L. (Ginseng) 2XFor temporary relief of tiredness, weakness and general fatigue - Uses
-
Warnings
Ask a doctor before use if you
- are taking minocycline, doxycycline, tetracycline or are on Coumadin therapy, as ginseng and zinc treatment may inhibit the absorption of these medicines.
- are under medical supervision for diabetes or have a skin condition called pemphigus vulgaris or have multiple sclerosis (MS), rheumatoid arthritis (RA), or an auto-immune condition.
Cold-EEZE® Plus Immune Support and Energy QuickMelts® is a combination product formulated to help you to reduce the duration of the common cold and its symptoms, promote immune system health and for temporary relief of tiredness, weakness and general fatigue. It is insufficient treatment for Influenza or Allergies.
-
Directions
- Take Cold-EEZE® Plus Immune Support and Energy QuickMelts® when you have cold symptoms.
- Adults:
- Dissolve entire tablet in mouth. Do not chew. Do not swallow whole.
- Take 1 tablet at the onset of symptoms.
- Repeat every 2-4 hours as needed until all symptoms subside.
- Do not eat or drink for 15 minutes after use, otherwise, drink plenty of fluids.
- Recommended daily dose is 6 tablets for adults.
- For individuals under the age of 18, consult a healthcare practitioner before use.
- Other information
- Other ingredients
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 24 Tablet Carton
NEW QuickMelts®
GREAT
TASTECold-EEZE®
Zinc Gluconate GlycineCOLD REMEDY
Plus
Natural
Immune Support
+NATURAL ENERGY
- ✓ Shortens Your Cold, Works Faster
- ✓ Promotes Immune Health,
Antioxidant Support- Rose Hips: Rich in Natural Vitamin C
- Echinacea
- ✓ Natural Energy
24
QuickMelts®
HomeopathicNatural
Orange Flavor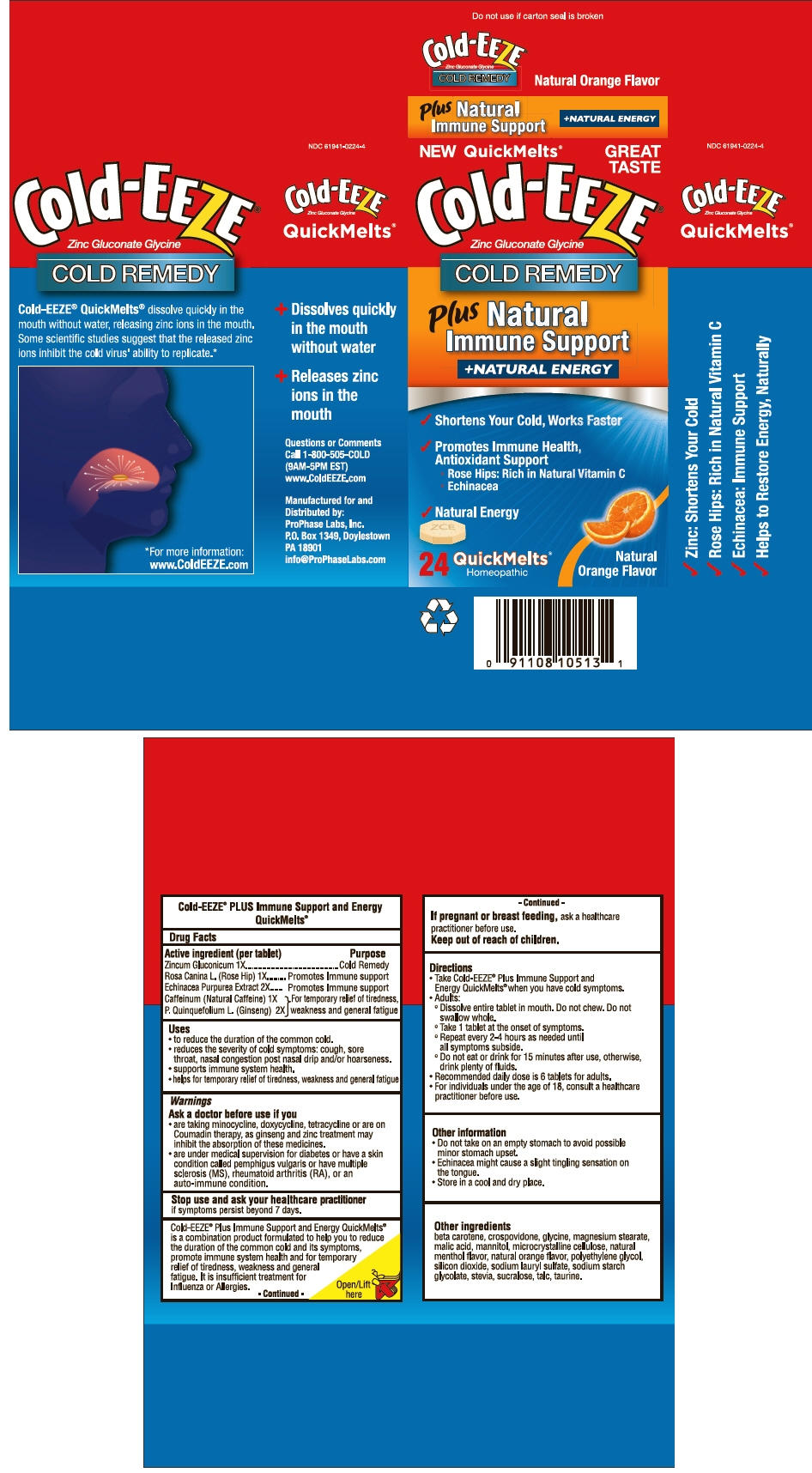
- ✓ Shortens Your Cold, Works Faster
-
INGREDIENTS AND APPEARANCE
COLD-EEZE PLUS IMMUNE SUPPORT PLUS ENERGY
zinc gluconate, rosa canina leaf, echinacea purpurea, caffeine, and panax notoginseng root tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61941-0224 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) ZINC GLUCONATE 1 [hp_X] ROSA CANINA LEAF (UNII: J3N2Z889QP) (ROSA CANINA LEAF - UNII:J3N2Z889QP) ROSA CANINA LEAF 1 [hp_X] ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 2 [hp_X] CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 1 [hp_X] PANAX NOTOGINSENG ROOT (UNII: GQX1C1175U) (PANAX NOTOGINSENG ROOT - UNII:GQX1C1175U) PANAX NOTOGINSENG ROOT 2 [hp_X] Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) .BETA.-CAROTENE (UNII: 01YAE03M7J) CROSPOVIDONE (UNII: 68401960MK) MAGNESIUM STEARATE (UNII: 70097M6I30) MALIC ACID (UNII: 817L1N4CKP) MANNITOL (UNII: 3OWL53L36A) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MENTHOL (UNII: L7T10EIP3A) ORANGE (UNII: 5EVU04N5QU) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEVIA REBAUDIUNA LEAF (UNII: 6TC6NN0876) SUCRALOSE (UNII: 96K6UQ3ZD4) TALC (UNII: 7SEV7J4R1U) TAURINE (UNII: 1EQV5MLY3D) Product Characteristics Color WHITE Score no score Shape OCTAGON (8 SIDED) Size 15mm Flavor ORANGE (NATURAL ORANGE FLAVOR) Imprint Code ZCE Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61941-0224-4 4 in 1 PACKAGE 1 NDC: 61941-0224-1 6 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 09/01/2013 08/31/2023 Labeler - ProPhase Labs, Inc. (620557298) Establishment Name Address ID/FEI Business Operations ProPhase Labs, Inc. 620557298 LABEL(61941-0224) , ANALYSIS(61941-0224) , REPACK(61941-0224) Establishment Name Address ID/FEI Business Operations Pharmaloz Manufacturing, Inc. 067101998 PACK(61941-0224) , REPACK(61941-0224) Establishment Name Address ID/FEI Business Operations Blispak, Inc. 194902235 MANUFACTURE(61941-0224) , ANALYSIS(61941-0224) , PACK(61941-0224)
Trademark Results [Cold-EEZE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COLD-EEZE 88294220 5839399 Live/Registered |
Mylan Consumer Healthcare, Inc. 2019-02-08 |
 COLD-EEZE 74405322 1838542 Live/Registered |
MYLAN CONSUMER HEALTHCARE INC. 1993-06-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.