VIGADRONE by Aucta Pharmaceuticals, Inc.
VIGADRONE by
Drug Labeling and Warnings
VIGADRONE by is a Prescription medication manufactured, distributed, or labeled by Aucta Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VIGADRONE- vigabatrin powder, for solution
Aucta Pharmaceuticals, Inc.
----------
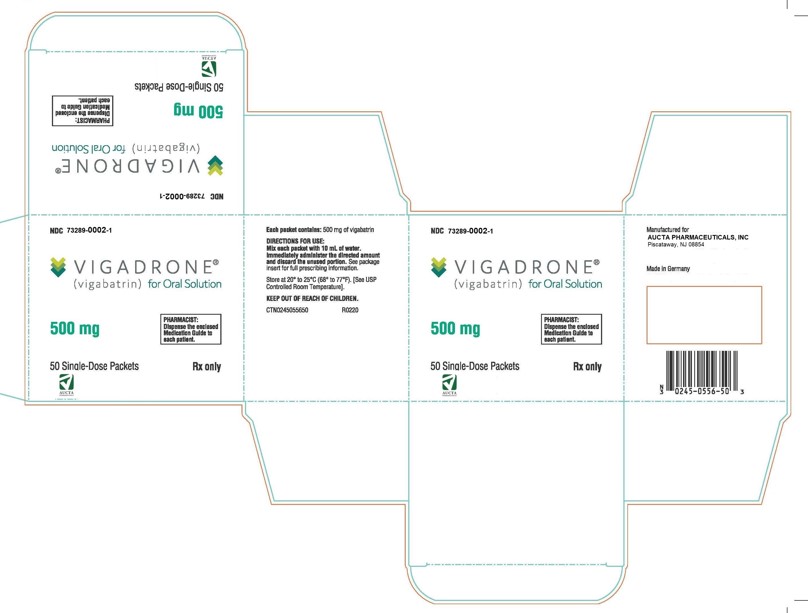
VIGADRONE® for oral solution is available as 500 mg packets containing a white to off-white granular powder. They are supplied in cartons of 50 packets (NDC: 73289-0002-1).
The oral syringes are provided separately by the pharmacy.
| VIGADRONE
vigabatrin powder, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Aucta Pharmaceuticals, Inc. (016894249) |
Revised: 2/2023
Document Id: f45b0470-8323-0154-e053-2995a90aa2d0
Set id: dd044b83-e2c8-66e8-e053-2a95a90aa3fd
Version: 3
Effective Time: 20230210
Trademark Results [VIGADRONE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VIGADRONE 97581463 not registered Live/Pending |
Upsher-Smith Laboratories, LLC 2022-09-07 |
 VIGADRONE 97581459 not registered Live/Pending |
Upsher-Smith Laboratories, LLC 2022-09-07 |
 VIGADRONE 87304605 5571338 Live/Registered |
UPSHER-SMITH LABORATORIES, LLC 2017-01-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.