CHLORDIAZEPOXIDE HYDROCHLORIDE capsule
Chlordiazepoxide Hydrochloride by
Drug Labeling and Warnings
Chlordiazepoxide Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Chartwell RX, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; AND DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (see WARNINGS AND PRECAUTIONS).
- The use of benzodiazepines, including chlordiazepoxide hydrochloride capsules, exposes users to risk of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing chlordiazepoxide hydrochloride capsules and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (see WARNINGS).
- The continued use of benzodiazepines, including chlordiazepoxide hydrochloride capsules, may lead to clinically significant physical dependence. The risk of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide hydrochloride capsules after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide hydrochloride capsules or reduce the dosage (see DOSAGE AND ADMINISTRATION and WARNINGS).
-
DESCRIPTION
Chlordiazepoxide Hydrochloride, is the prototype for the benzodiazepine compounds. It is a versatile therapeutic agent of proven value for the relief of anxiety. Chlordiazepoxide Hydrochloride is among the safer of the effective psychopharmacologic compounds available, as demonstrated by extensive clinical evidence.
Chlordiazepoxide Hydrochloride is available as capsules containing 5 mg, 10 mg or 25 mg chlordiazepoxide hydrochloride. In addition, each capsule contains the following inactive ingredients:
5 mg: colloidal silicon dioxide, corn starch, D & C Yellow #10, FD & C Blue #1, FD & C Yellow #6, gelatin, lactose monohydrate, talc, titanium dioxide, shellac, black iron oxide, and propylene glycol.
10 mg: colloidal silicon dioxide, corn starch, D & C Yellow #10, FD & C Blue #1, FD & C Red #3, FD & C Yellow #6, gelatin, lactose monohydrate, talc, titanium dioxide, shellac, propylene glycol, and simethicone.
25 mg: colloidal silicon dioxide, corn starch, D & C Yellow #10, FD & C Blue #1, gelatin, lactose monohydrate, talc, titanium dioxide, shellac, black iron oxide, and propylene glycol.
Chlordiazepoxide Hydrochloride is 7-chloro-2-(methylamino)-5-phenyl-3H-1,4-benzodiazepine 4-oxide hydrochloride. A white to practically white crystalline substance, it is soluble in water. It is unstable in solution and the powder must be protected from light. The molecular weight is 336.22. The structural formula of chlordiazepoxide hydrochloride is as follows:
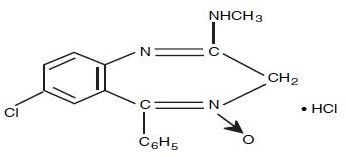
-
CLINICAL PHARMACOLOGY
Chlordiazepoxide Hydrochloride has antianxiety, sedative, appetite-stimulating and weak analgesic actions. The precise mechanism of action is not known. The drug blocks EEG arousal from stimulation of the brain stem reticular formation. It takes several hours for peak blood levels to be reached and the half-life of the drug is between 24 and 48 hours. After the drug is discontinued plasma levels decline slowly over a period of several days. Chlordiazepoxide is excreted in the urine, with 1% to 2% unchanged and 3% to 6% as conjugate.
Animal Pharmacology
The drug has been studied extensively in many species of animals and these studies are suggestive of action on the limbic system of the brain, which recent evidence indicates is involved in emotional responses.
Hostile monkeys were made tame by oral drug doses which did not cause sedation. Chlordiazepoxide Hydrochloride revealed a “taming” action with the elimination of fear and aggression. The taming effect of chlordiazepoxide hydrochloride was further demonstrated in rats made vicious by lesions in the septal area of the brain. The drug dosage which effectively blocked the vicious reaction was well below the dose which caused sedation in these animals.
The LD 50 of parenterally administered chlordiazepoxide hydrochloride was determined in mice (72 hours) and rats (5 days), and calculated according to the method of Miller and Tainter, with the following results: mice, IV, 123±12mg/kg; mice, IM, 366±7mg/kg; rats, IV, 120±7 mg/kg; rats, IM, >160 mg/kg.
Effects on Reproduction
Reproduction studies in rats fed 10, 20 and 80 mg/kg daily and bred through one or two matings showed no congenital anomalies, nor were there adverse effects on lactation of the dams or growth of the newborn. However, in another study at 100 mg/kg daily there was noted a significant decrease in the fertilization rate and a marked decrease in the viability and body weight of offspring which may be attributable to sedative activity, thus resulting in lack of interest in mating and lessened maternal nursing and care of the young. One neonate in each of the first and second matings in the rat reproduction study at the 100 mg/kg dose exhibited major skeletal defects. Further studies are in progress to determine the significance of these findings.
-
INDICATIONS AND USAGE
Chlordiazepoxide Hydrochloride Capsules are indicated for the management of anxiety disorders or for the short term relief of symptoms of anxiety, withdrawal symptoms of acute alcoholism, and preoperative apprehension and anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The effectiveness of chlordiazepoxide hydrochloride capsules in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
- CONTRAINDICATIONS
-
WARNINGS
Risks from Concomitant Use with Opioids:
Concomitant use of benzodiazepines, including chlordiazepoxide, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe chlordiazepoxide concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of chlordiazepoxide than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking chlordiazepoxide, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when chlordiazepoxide is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see PRECAUTIONS: Drug Interactions).
Abuse, Misuse, and Addiction:
The use of benzodiazepines, including chlordiazepoxide, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE: Abuse).
Before prescribing chlordiazepoxide and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of chlordiazepoxide, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of chlordiazepoxide along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal Reactions:
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of Chlordiazepoxide).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines, including chlordiazepoxide, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE: Dependence).
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE: Dependence).
Chlordiazepoxide Hydrochloride may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a vehicle or operating machinery. Similarly, it may impair mental alertness in children. The concomitant use of alcohol or other central nervous system depressants may have an additive effect. PATIENTS SHOULD BE WARNED ACCORDINGLY.
Neonatal Sedation and Withdrawal Syndrome
Use of chlordiazepoxide hydrochloride late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate ( see PRECAUTIONS, Pregnancy). Monitor neonates exposed to Chlordiazepoxide hydrochloride during pregnancy or labor for signs of sedation and monitor neonates exposed to chlordiazepoxide hydrochloride during pregnancy for signs of withdrawal; manage these infants accordingly. -
PRECAUTIONS
In elderly and debilitated patients, it is recommended that the dosage be limited to the smallest effective amount to preclude the development of ataxia or oversedation (10 mg or less per day initially, to be increased gradually as needed and tolerated). In general, the concomitant administration of chlordiazepoxide and other psychotropic agents is not recommended. If such combination therapy seems indicated, careful consideration should be given to the pharmacology of the agents to be employed particularly when the known potentiating compounds such as MAO inhibitors and phenothiazines are to be used. The usual precautions in treating patients with impaired renal or hepatic function should be observed.
Paradoxical reactions, e.g., excitement, stimulation and acute rage, have been reported in psychiatric patients and in hyperactive aggressive pediatric patients, and should be watched for during chlordiazepoxide therapy. The usual precautions are indicated when chlordiazepoxide is used in the treatment of anxiety states where there is any evidence of impending depression; it should be borne in mind that suicidal tendencies may be present and protective measures may be necessary. Although clinical studies have not established a cause and effect relationship, physicians should be aware that variable effects on blood coagulation have been reported very rarely in patients receiving oral anticoagulants and chlordiazepoxide. In view of isolated reports associating chlordiazepoxide with exacerbation of porphyria, caution should be exercised in prescribing chlordiazepoxide to patients suffering from this disease.
Pediatric Use
Because of the varied response of pediatric patients to CNS-acting drugs, therapy should be initiated with the lowest dose and increased as required (see DOSAGE AND ADMINISTRATION). Since clinical experience with chlordiazepoxide in pediatric patients under 6 years of age is limited, use in this age group is not recommended. Hyperactive aggressive pediatric patients should be monitored for paradoxical reactions to chlordiazepoxide (see PRECAUTIONS).
Information for Patients
Advise the patient to read the FDA-approved patient labeling ( Medication Guide).
Risks from Concomitant Use with Opioids
Advise both patients and caregivers about the risk of potentially fatal respiratory depression and sedation when chlordiazepoxide is used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see WARNINGS: Risks from Concomitant Use with Opioids and PRECAUTIONS: Drug Interactions).
Abuse, Misuse, and Addiction
Inform patients that the use of chlordiazepoxide, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug (see WARNINGS: Abuse, Misuse, and Addiction and DRUG ABUSE AND DEPENDENCE).
Withdrawal Reactions
Inform patients that the continued use of chlordiazepoxide may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of chlordiazepoxide may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of chlordiazepoxide may require a slow taper (see WARNINGS: Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE).
Pregnancy
Advise pregnant females that use of chlordiazepoxide hydrochloride late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns (see Warnings, Neonatal Sedation and Withdrawal Syndrome and Precautions, Pregnancy).
Instruct patients to inform their healthcare provider if they are pregnant.
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to chlordiazepoxide hydrochloride during pregnancy ( see Precautions, Pregnancy) .
Nursing
Advise patients that breastfeeding is not recommended during treatment with chlordiazepoxide hydrochloride (see Precautions, Nursing Mothers).Drug Interactions
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists.
Limit dosage and duration of concomitant use of benzodiazepines and opioids, and monitor patients closely for respiratory depression and sedation.
Pregnancy:
Pregnancy Exposure Registry
There is a pregnancy registry that monitors pregnancy outcomes in women exposed to psychiatric medications, including chlordiazepoxide hydrochloride, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/pregnancyregistry/.
Risk Summary
Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal (see WARNINGS, Neonatal Sedation and Withdrawal Syndrome, and Clinical Considerations). Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects ( see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia and sedation in neonates. Monitor neonates exposed to chlordiazepoxide hydrochloride during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems Monitor neonates exposed to chlordiazepoxide hydrochloride during pregnancy for signs of withdrawal. Manage these neonates accordingly (see WARNINGS, Neonatal Sedation and Withdrawal Syndrome).
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Nursing:
Risk Summary
There are no data on the presence of chlordiazepoxide in human milk. There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. Because of chlordiazepoxide’s long half-life, the potential for chlordiazepoxide to accumulate in breast milk and the potential for serious adverse reactions, including sedation and withdrawal symptoms in breastfed infants, advise patients that breastfeeding is not recommended during treatment with chlordiazepoxide hydrochloride. -
ADVERSE REACTIONS
The necessity of discontinuing therapy because of undesirable effects has been rare. Drowsiness, ataxia and confusion have been reported in some patients particularly the elderly and debilitated. While these effects can be avoided in almost all instances by proper dosage adjustment, they have occasionally been observed at the lower dosage ranges.
In a few instances syncope has been reported.
Other adverse reactions reported during therapy include isolated instances of skin eruptions, edema, minor menstrual irregularities, nausea and constipation, extrapyramidal symptoms, as well as increased and decreased libido. Such side effects have been infrequent, and are generally controlled with reduction of dosage. Changes in EEG patterns (low-voltage fast activity) have been observed in patients during and after chlordiazepoxide treatment.
Blood dyscrasias (including agranulocytosis), jaundice and hepatic dysfunction have occasionally been reported during therapy. When chlordiazepoxide treatment is protracted, periodic blood counts and liver function tests are advisable.
To report SUSPECTED ADVERSE REACTIONS, contact Chartwell RX, LLC. at 1-845-232-1683 or FDA at 1- 800-FDA-1088 or www.fda.gov/medwatch.
-
DRUG ABUSE AND DEPENDENCE
Controlled Substance
Chlordiazepoxide is a Schedule IV controlled substance.
Abuse
Chlordiazepoxide is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a healthcare provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties In controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medications. Abuse and misuse may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders (see WARNINGS: Abuse, Misuse, and Addiction).
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
Dependence
Physical Dependence
Chlordiazepoxide may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use (see WARNINGS: Dependence and Withdrawal Reactions).
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide or reduce the dosage (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of Chlordiazepoxide and WARNINGS: Dependence and Withdrawal Reactions).
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizure, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
Tolerance
Tolerance to chlordiazepoxide may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effects of chlordiazepoxide may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
Management of Overdose
Deaths may occur from overdosage with this class of drugs. Multiple drug ingestion (including alcohol) is common in deliberate tricyclic antidepressant overdose. As the management is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity develop rapidly after tricyclic antidepressant overdose; therefore, hospital monitoring is required as soon as possible.
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal (see WARNINGS: Abuse, Misuse, and Addiction). Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway management. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting a poison center (1-800-222-1222), poisoncontrol.org, or medical toxicologist for additional overdosage management recommendations. -
DOSAGE AND ADMINISTRATION
Because of the wide range of clinical indications for chlordiazepoxide, the optimum dosage varies with the diagnosis and response of the individual patient. The dosage, therefore, should be individualized for maximum beneficial effects.
Adults Usual Daily Dose
Relief of Mild and Moderate Anxiety Disorders and Symptoms of Anxiety 5 mg or 10 mg, 3 or 4 times daily Relief of Severe Anxiety Disorders and Symptoms of Anxiety
20 mg or 25 mg, 3 or 4 times daily
Geriatric Patients, or in the presence of debilitating disease
5 mg, 2 to 4 times daily
Preoperative Apprehension and Anxiety: On days preceding surgery, 5 to 10 mg orally, 3 or 4 times daily. If used as preoperative medication, 50 to 100 mg IM* 1 hour prior to surgery PEDIATRIC PATIENTS USUAL DAILY DOSE Because of the varied response of pediatric patients to CNS-acting drugs, therapy should be initiated with the lowest dose and increased as required.
Since clinical experience in pediatric patients under 6 years of age is limited, the use of the drug in this age group is not recommended.5 mg, 2 to 4 times daily (may be increased in some pediatric patients to 10 mg, 2 to 3 times daily) For the relief of withdrawal symptoms of acute alcoholism, the parenteral form* is usually used initially. If the drug is administered orally, the suggested initial dose is 50 to 100 mg, to be followed by repeated doses as needed until agitation is controlled - up to 300 mg per day. Dosage should then be reduced to maintenance levels.
*See package insert for Injectable Chlordiazepoxide Hydrochloride
Discontinuation or Dosage Reduction of Chlordiazepoxide
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increase the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see WARNINGS: Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE: Dependence). -
HOW SUPPLIED
Chlordiazepoxide Hydrochloride Capsules, USP are available in the following presentations:
5 mg:
Light green and yellow, size #4 hard gelatin capsules, filled with white to off-white powder, imprinted “CE” on the cap and “81” on the body in black ink. Bottles of 60 (NDC: 62135-220-60)
10 mg:
Black and green, size #4 hard gelatin capsules, filled with white to off-white powder, imprinted “CE” on the cap and “82” on the body in white ink. Bottles of 60 (NDC: 62135-221-60)
25 mg:
Light green and white, size #4 hard gelatin capsules, filled with white to off-white powder, imprinted “CE” on the cap and “83” on the body in black ink. Bottles of 60 (NDC: 62135-222-60)
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Manufactured by:
CorePharma, LLC
Middlesex, NJ 08846Manufactured for:
Chartwell RX, LLC
Congers, NY 10920L70777 Rev 11/2022
Dispense with Medication Guide available at www.chartwellpharma.com/medguides
-
Medication Guide
Medication Guide
Chlordiazepoxide Hydrochloride(klor″ dye az″ e pox′ ide hye″droe klor′ ide) Capsules, USP C-IV
What is the most important information I should know about chlordiazepoxide hydrochloride?
-
Chlordiazepoxide Hydrochloride is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system depressants (CNS) (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death. Get emergency help right away if any of the following happens:
- shallow or slowed breathing
- breathing stops (which may lead to the heart stopping)
- excessive sleepiness (sedation)
Do not drive or operate heavy machinery until you know how taking chlordiazepoxide hydrochloride with opioids affects you.
-
Risk of abuse, misuse, and addiction. There is a risk of abuse, misuse, and addiction with benzodiazepines, including chlordiazepoxide hydrochloride, which can lead to overdose and serious side effects including coma and death.
- Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including chlordiazepoxide hydrochloride. These serious side effects may also include delirium, paranoia, suicidal thoughts or actions, seizures, and difficulty breathing. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects.
- You can develop an addiction even if you take chlordiazepoxide hydrochloride exactly as prescribed by your healthcare provider.
- Take chlordiazepoxide hydrochloride exactly as your healthcare provider prescribed.
- Do not share your chlordiazepoxide hydrochloride with other people.
- Keep chlordiazepoxide hydrochloride in a safe place and away from children.
-
Physical dependence and withdrawal reactions. Chlordiazepoxide Hydrochloride can cause physical dependence and withdrawal reactions, especially if you continue to take chlordiazepoxide hydrochloride for several days to several weeks.
- Do not suddenly stop taking chlordiazepoxide hydrochloride. Stopping chlordiazepoxide hydrochloride suddenly can cause serious and life-threatening side effects, including unusual movements, responses, or expressions, seizures, sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these symptoms.
- Some people who suddenly stop benzodiazepines have symptoms that can last for several weeks to more than 12 months, including, anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle twitching, burning or prickling feeling in your hands, arms, legs or feet, and ringing in your ears.
- Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.
- Do not take more chlordiazepoxide hydrochloride than prescribed or take chlordiazepoxide hydrochloride for longer than prescribed.
What is chlordiazepoxide hydrochloride?
- Chlordiazepoxide Hydrochloride is a prescription medicine used:
- to treat anxiety disorders
- for the short-term relief of the symptoms of anxiety
- to treat withdrawal symptoms of acute alcoholism
- preoperatively to treat apprehension and anxiety
- Chlordiazepoxide Hydrochloride is a federal controlled substance (C-IV) because it contains chlordiazepoxide that can be abused or lead to dependence. Keep chlordiazepoxide hydrochloride in a safe place to prevent misuse and abuse. Selling or giving away chlordiazepoxide may harm others, and is against the law. Tell your healthcare provider if you have abused or been dependent on alcohol, prescription medicines or street drugs.
- It is not known if chlordiazepoxide is safe and effective in children under 6 years of age.
- It is not known if chlordiazepoxide is safe and effective for use for longer than 4 months.
Do not take chlordiazepoxide hydrochloride if you are allergic to chlordiazepoxide or to any of the ingredients in chlordiazepoxide hydrochloride capsules. See the end of this Medication Guide for a complete list of ingredients in chlordiazepoxide hydrochloride capsules.
Before you take chlordiazepoxide hydrochloride, tell your healthcare provider about all of your medical conditions, including if you:
have or have had depression, mood problems, or suicidal thoughts or behavior
have a history of drug or alcohol abuse or addiction
have liver or kidney problemsare pregnant or plan to become pregnant.
o Taking chlordiazepoxide hydrochloride late in pregnancy may cause your baby to have symptoms of sedation (breathing problems, sluggishness, low muscle tone), and/or withdrawal symptoms (jitteriness, irritability, restlessness, shaking, excessive crying, feeding problems).
o Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with chlordiazepoxide hydrochloride.
o There is a pregnancy registry for women who take chlordiazepoxide hydrochloride during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. If you become pregnant during treatment with chlordiazepoxide hydrochloride, talk to your healthcare
provider about registering with the National Pregnancy Registry for Psychiatric Medications. You can register by calling 1-866-961-2388 or visiting
https://womansmentalhealth.org/pregnancyregistry/.are breastfeeding or plan to breastfeed. Chlordiazepoxide hydrochloride may pass into your breast milk.
o Talk to your healthcare provider about the best way to feed your baby if you take chlordiazepoxide hydrochloride.
o Breastfeeding is not recommended during treatment with chlordiazepoxide hydrochloride.Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking chlordiazepoxide hydrochloride with certain other medicines can cause side effects or affect how well chlordiazepoxide hydrochloride or the other medicines work.
Ask your healthcare provider if you are not sure if you are taking any of these medicines. Your healthcare provider can tell you if it is safe to take chlordiazepoxide hydrochloride with your other medicines.
Do not start or stop any other medicines during treatment with chlordiazepoxide hydrochloride without talking to your healthcare provider first. Stopping chlordiazepoxide hydrochloride suddenly may cause you to have serious side effects. See, “What are the possible side effects of chlordiazepoxide hydrochloride?”
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take chlordiazepoxide?
- Take chlordiazepoxide exactly as your healthcare provider tells you to take it.
- If you take too much chlordiazepoxide hydrochloride, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of chlordiazepoxide hydrochloride? Chlordiazepoxide hydrochloride may cause serious side effects, including:
- See “What is the most important information I should know about chlordiazepoxide hydrochloride?”
-
Chlordiazepoxide Hydrochloride can make you sleepy or dizzy and can slow your thinking and motor skills.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how chlordiazepoxide hydrochloride affects you.
- Do not drink alcohol or take other drugs that may make you sleepy or dizzy while taking chlordiazepoxide hydrochloride without first talking to your healthcare provider. When taken with alcohol or drugs that cause sleepiness or dizziness, chlordiazepoxide hydrochloride may make your sleepiness or dizziness much worse.
The most common side effects of chlordiazepoxide hydrochloride include:
- drowsiness
- loss of control of body movements (ataxia)
- confusion
These are not all the possible side effects of chlordiazepoxide hydrochloride. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store chlordiazepoxide hydrochloride?
- Store chlordiazepoxide hydrochloride at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep chlordiazepoxide hydrochloride in a tightly closed container and out of the light.
Keep chlordiazepoxide hydrochloride and all medicines out of the reach of children.
General information about the safe and effective use of chlordiazepoxide hydrochloride.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use chlordiazepoxide hydrochloride for a condition for which it was not prescribed. Do not give chlordiazepoxide hydrochloride to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about chlordiazepoxide hydrochloride that is written for health professionals.
What are the ingredients in chlordiazepoxide hydrochloride?
Active ingredient: chlordiazepoxide hydrochloride
Inactive ingredients:
5 mg: colloidal silicon dioxide, corn starch, D & C Yellow #10, FD & C Blue #1, FD & C Yellow #6, gelatin, lactose monohydrate, talc, titanium dioxide, shellac, black iron oxide, and propylene glycol.
10 mg: colloidal silicon dioxide, corn starch, D & C Yellow #10, FD & C Blue #1, FD & C Red #3, FD & C Yellow #6, gelatin, lactose monohydrate, talc, titanium dioxide, shellac, propylene glycol, and simethicone.
25 mg: colloidal silicon dioxide, corn starch, D & C Yellow #10, FD & C Blue #1, gelatin, lactose monohydrate, talc, titanium dioxide, shellac, black iron oxide, and propylene glycol.
Manufactured by:
CorePharma, LLC
Middlesex, NJ 08846
Manufactured for:Chartwell RX, LLC
Congers, NY 10920
For more information, contact Chartwell RX, LLC. at 1-845-232-1683
This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 08/2022
L70778
-
Chlordiazepoxide Hydrochloride is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system depressants (CNS) (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death. Get emergency help right away if any of the following happens:
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Chlordiazepoxide Hydrochloride 5mg - NDC: 62135-220-60 - 60 Capsules Label

Chlordiazepoxide Hydrochloride 10mg - NDC: 62135-221-60 - 60 Capsules Label

Chlordiazepoxide Hydrochloride 25mg - NDC: 62135-222-60 - 60 Capsules Label

-
INGREDIENTS AND APPEARANCE
CHLORDIAZEPOXIDE HYDROCHLORIDE
chlordiazepoxide hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62135-220 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORDIAZEPOXIDE HYDROCHLORIDE (UNII: MFM6K1XWDK) (CHLORDIAZEPOXIDE - UNII:6RZ6XEZ3CR) CHLORDIAZEPOXIDE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color green (light green/ yellow) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code CE;81 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62135-220-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 04/18/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA084678 06/15/1976 CHLORDIAZEPOXIDE HYDROCHLORIDE
chlordiazepoxide hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62135-221 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORDIAZEPOXIDE HYDROCHLORIDE (UNII: MFM6K1XWDK) (CHLORDIAZEPOXIDE - UNII:6RZ6XEZ3CR) CHLORDIAZEPOXIDE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color green (black/green) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code CE;82 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62135-221-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 04/18/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA084041 06/15/1976 CHLORDIAZEPOXIDE HYDROCHLORIDE
chlordiazepoxide hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62135-222 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORDIAZEPOXIDE HYDROCHLORIDE (UNII: MFM6K1XWDK) (CHLORDIAZEPOXIDE - UNII:6RZ6XEZ3CR) CHLORDIAZEPOXIDE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color green (light green/white) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code CE;83 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62135-222-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 04/18/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA084679 06/15/1976 Labeler - Chartwell RX, LLC (079394054)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.