COTZ FACE (NATURAL SKIN TONE) SPF 40 SUNSCREEN- titanium dioxide, zinc oxide cream
Cotz Face (Natural Skin Tone) SPF 40 by
Drug Labeling and Warnings
Cotz Face (Natural Skin Tone) SPF 40 by is a Otc medication manufactured, distributed, or labeled by Fallien Cosmeceuticals, LTD., Custom Analytics LLC, Process Technologies & Packaging. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredients:
- Purpose
-
Uses:
Helps prevent sunburn. If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings:
-
Directions:
- Apply liberally 15 mtnutes before sun exposure.
- Reapply:
- - After 80 minutes of swimming or sweating
- - Immediately after towel drying
- - At least every 2 hours
- Children under 6 months: Ask a doctor
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures Including:
- - Limit time in the sun, especially from 10 am-2 p.m.
- - Wear long-sleeved shirts, pants, hats, and sunglasses
- Inactive Ingredients:
- Other Information:
-
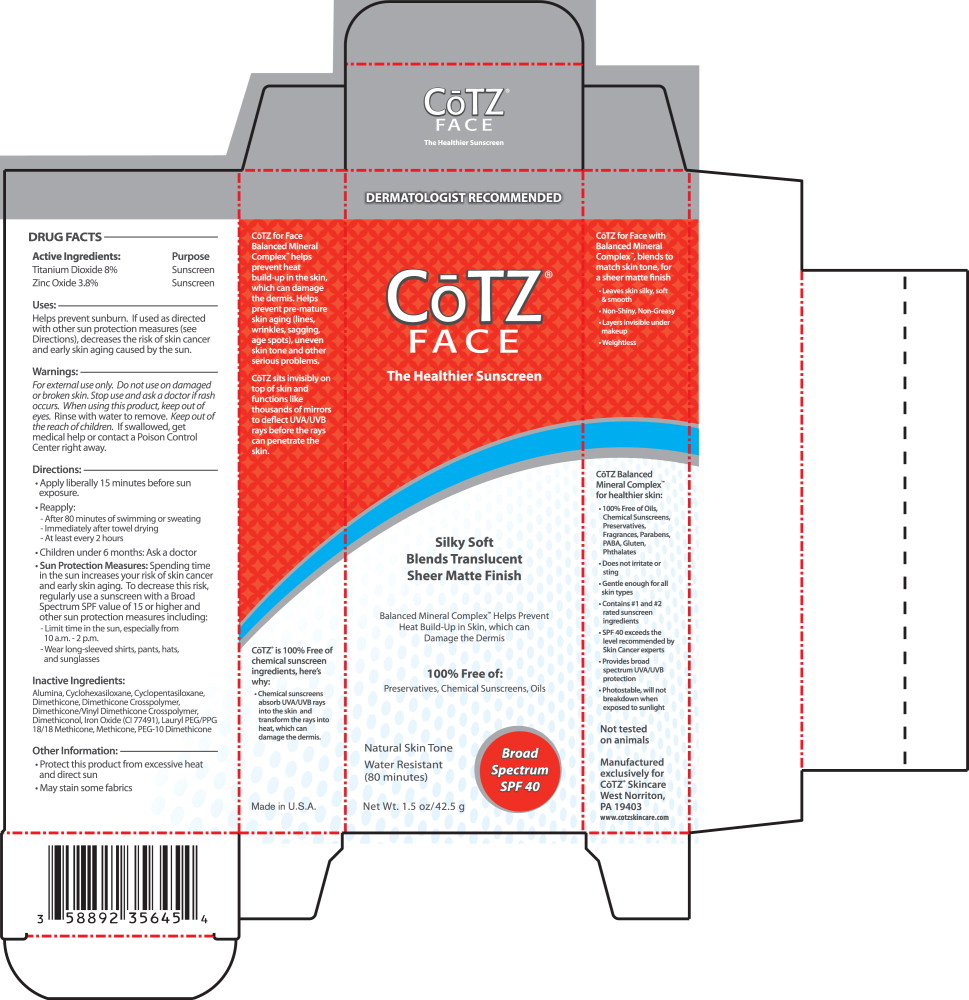
PRINCIPAL DISPLAY PANEL
Principal Display Panel – Carton Label
DERMATOLOGIST RECOMMENDED
CōTZ®
FACE
The Healthier Sunscreen
Silky Soft
Blends Translucent
Sheer Matte FinishBalanced Mineral Complex™ Helps Prevent
Heat Build-Up in Skin, which can
Damage the Dermis100% Free of:
Preservatives, Chemical Sunscreens, OilsNatural Skin Tone
Water Resistant
(80 minutes)Broad
Spectrum
SPF 40Net Wt. 1.5 oz/ 42.5 g
-
INGREDIENTS AND APPEARANCE
COTZ FACE (NATURAL SKIN TONE) SPF 40 SUNSCREEN
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58892-356 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 80 mg in 1 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 38 mg in 1 g Inactive Ingredients Ingredient Name Strength Aluminum Oxide (UNII: LMI26O6933) Cyclomethicone 6 (UNII: XHK3U310BA) Cyclomethicone 5 (UNII: 0THT5PCI0R) Dimethicone 20 (UNII: H8YMB5QY0D) Dimethicone/Diene Dimethicone Crosspolymer (UNII: RSA9I561OK) Dimethiconol (100000 cst) (UNII: OSA9UP217S) Ferric Oxide Red (UNII: 1K09F3G675) Lauryl PEG/PPG-18/18 Methicone (UNII: ZJ5S27D9NX) Methicone (20 cst) (UNII: 6777U11MKT) PEG-10 Dimethicone (600 cst) (UNII: 8PR7V1SVM0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58892-356-45 1 in 1 CARTON 1 42.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/14/2012 Labeler - Fallien Cosmeceuticals, LTD. (958388357) Establishment Name Address ID/FEI Business Operations Custom Analytics LLC 144949372 ANALYSIS(58892-356) Establishment Name Address ID/FEI Business Operations Process Technologies & Packaging 809172885 MANUFACTURE(58892-356)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.