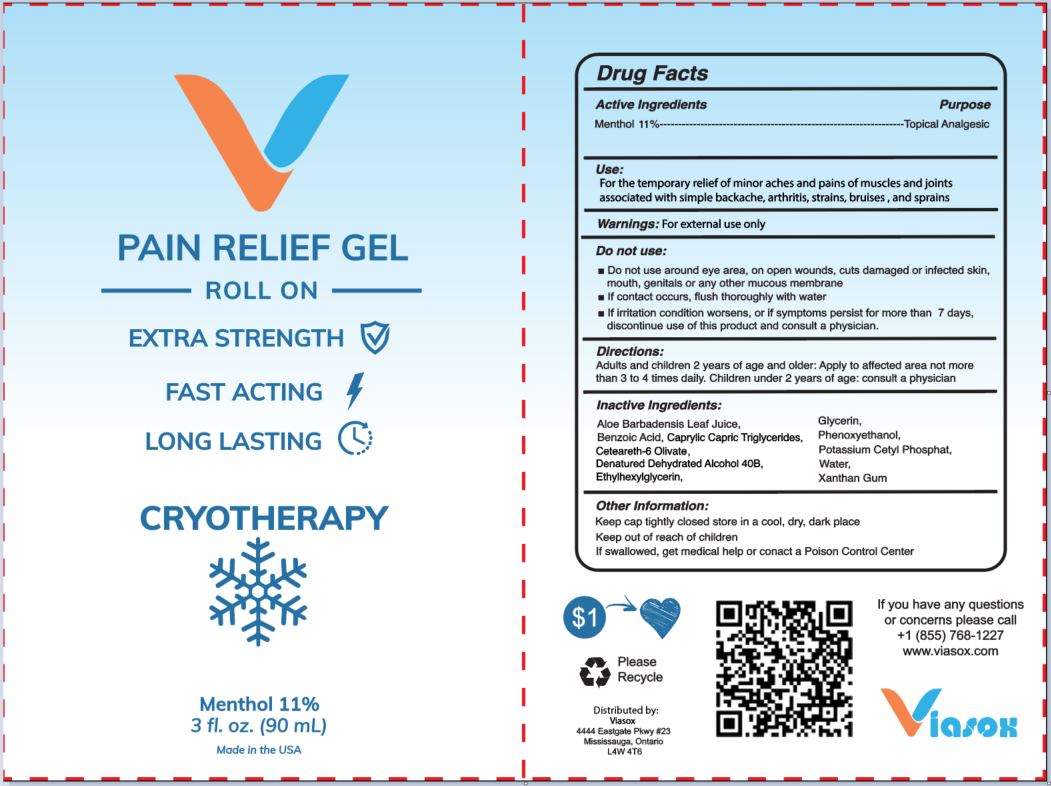
VIASOX (as PLD) - PAIN RELIEF GEL (ROLL ON) (82687-101) - DELIST
PAIN RELIEF by
Drug Labeling and Warnings
PAIN RELIEF by is a Otc medication manufactured, distributed, or labeled by VIASOX LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PAIN RELIEF ROLL ON- menthol gelÂ
VIASOX LTD
----------
VIASOX (as PLD) - PAIN RELIEF GEL (ROLL ON) (82687-101) - DELIST
USE
FOR THE TEMPORARY RELIEF OF MINOR ACHES AND PAINS OF MUSCLES AND JOINTS ASSOCIATED WITH SIMPLE BACKACHE, STRAINS, BRUISES, AND SPRAINS
WARNINGS
FOR EXTERNAL USE ONLY.
DO NOT USE:
- DO NOT USE AROUND EYE AREA, ON OPEN WOUNDS, CUTS, DAMAGED OR INFECTED SKIN, MOUTH, GENITALS OR ANY OTHER MUCOUS MEMBRANE
- IF CONTACT OCCURS, FLUSH THOROUGHLY WITH WATER
- IF IRRITATION CONDITION WORSENS, OR IF SYMPTOMS PERSIST FOR MORE THAN 7 DAYS, DISCONTINUE USE OF THIS PRODUCT AND CONSULT A PHYSICIAN.
DIRECTIONS:
ADULTS AND CHILDREN 2 YEARS OF AGE AND OLDER: APPLY TO AFFECTED AREA NOT MORE THAN 3 TO 4 TIMES DAILY. CHILDREN UNDER 2 YEARS OF AGE: CONSULT A PHYSICIAN.
| PAIN RELIEFÂ
ROLL ON
menthol gel |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler -Â VIASOX LTD (240308383) |
Revised: 3/2024
Â
Document Id: 14bc78f3-c974-d45f-e063-6294a90aa9e5
Set id: dd949b29-e5ba-6e0d-e053-2995a90a6025
Version: 3
Effective Time: 20240328
Trademark Results [PAIN RELIEF]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PAIN RELIEF 97238381 not registered Live/Pending |
Liu, Caiqing 2022-01-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.