Derpixa by Scite Pharma, LLC DERPIXA-
Derpixa by
Drug Labeling and Warnings
Derpixa by is a Other medication manufactured, distributed, or labeled by Scite Pharma, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
Description
Derpixa is an occlusive, non-resorbable, self-drying and transparent gel.
When used as directed Derpixa dries to form a protective layer that is gas permeable and waterproof which hydrates and protects compromised skin areas and superficial wounds from chemical and microbial invasion.
Derpixa helps to promote a moist healing environment.
This moist wound healing environment promotes faster re-epithelialization1and reduces the skin's acute inflammatory response.
Derpixa is suitable for large surface areas and contoured skin like head, face, hand and foot, as well as joints and hairy areas without the need for shaving.
- 1 Losi P et al. J Mater Sci Mater Med.2012;23(9):2235–43
-
Indication for use
Derpixa is intended to be used under the direction of healthcare practitioners in the management of cutaneous reactions.
Derpixa is indicated for use on all types of wounds, toxic and compromised skin including:
- Cutaneous reactions
- Pruritic, itchy skin
- Xerotic, dry skin
- Desquamation
- Fissures of skin and nail folds
- Blisters
- Medical Adhesive-Related Skin Injuries (MARSI)
- Erythema
- Infusion reactions
- Rashes, including: Maculopapular rash, hand-foot syndrome, GVHD, acneiform reaction, peri – and appendageal (hair follicles, sweat glands)
Derpixa is indicated for the relief of dry, itching, flaking, peeling and irritated skin, as well as the symptomatic relief of pain, redness and heat sensation.
Derpixa may be directly applied to dry skin, open wounds and compromised or desquamating skin surfaces, including cutaneous rashes.
Derpixa gel is bacteriostatic and inert.
It contains no alcohols, parabens or fragrances.
Derpixa can be used with or without a secondary protective dressing.
Derpixa is suitable for children and people with sensitive skin.
Derpixa is intended for single patient use.
-
Directions for use
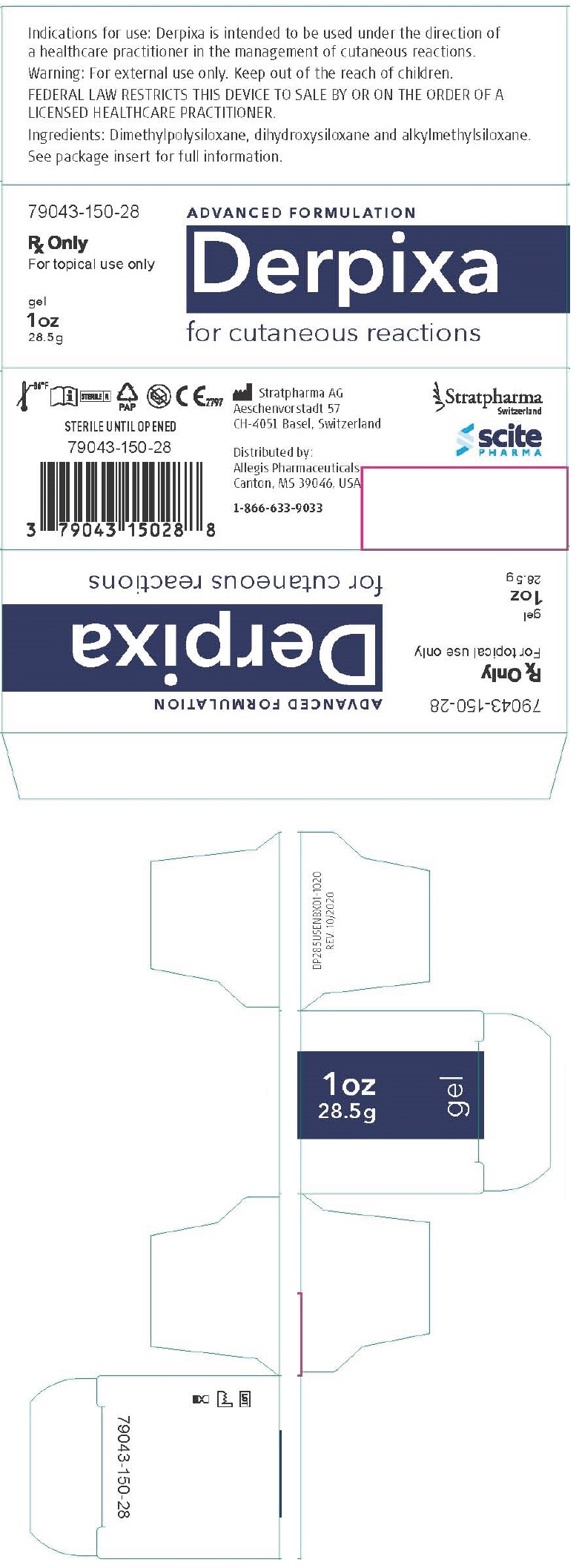
On the first use of 1 oz (28.5 g) tubes remove the cap, then the protective seal and close with the cap after use. Ensure that the affected superficial area is clean and dry.
Apply a very thin layer of Derpixa directly to the affected area and allow the gel to dry.
When applied correctly to exposed areas, Derpixa should be dry in 5–6 minutes.
If it takes longer to dry you have probably applied too much.
Gently remove the excess with a clean tissue or gauze and allow the drying process to continue.
Once dry, Derpixa may be covered by sunscreen, cosmetics and clothing.
Derpixa should be applied at least twice daily to affected areas, as needed or as advised by your physician.
For best results Derpixa should be maintained in continuous contact with the skin (24 hours a day/7 days a week).
How much Derpixa do I need?
Derpixa gel is an advanced formulation that requires substantially less product per application than typical creams or gels.
Derpixa 1 oz (28.5 g) is enough to treat an area of 6 × 12 inch (15 × 30 cm) twice per day for 30 days.
-
Warning
- For external use only.
- Derpixa should not be placed in contact with the eyes.
- Derpixa should not be applied over topical medications unless advised by your physician.
- Derpixa may stain clothing if not completely dry. If staining occurs, dry cleaning should be able to remove it without damaging the fabric.
- For correct storage please reclose the tube tightly with the cap.
- If irritation occurs, discontinue use and consult your physician.
- Keep out of the reach of children.
- Do not use after the expiration (EXP) date printed on the tube. The expiration (EXP) date does not change once the tube has been opened.
- Do not use if the tube is damaged.
- Contraindications
- STERILE UNTIL OPENED
- How supplied
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 28.5 g Tube Box
-
INGREDIENTS AND APPEARANCE
DERPIXA
dressing, wound, occlusiveProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:79043-150 Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONOL (2000 CST) (UNII: T74O12AN6Y) DIDECYLDIMONIUM CHLORIDE (UNII: JXN40O9Y9B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:79043-150-28 1 in 1 BOX 1 28.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Exempt device NAD 12/01/2025 Labeler - Scite Pharma, LLC (117555106)
Trademark Results [Derpixa]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DERPIXA 90322356 not registered Live/Pending |
Velocity Consulting Specialist, LLC 2020-11-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.