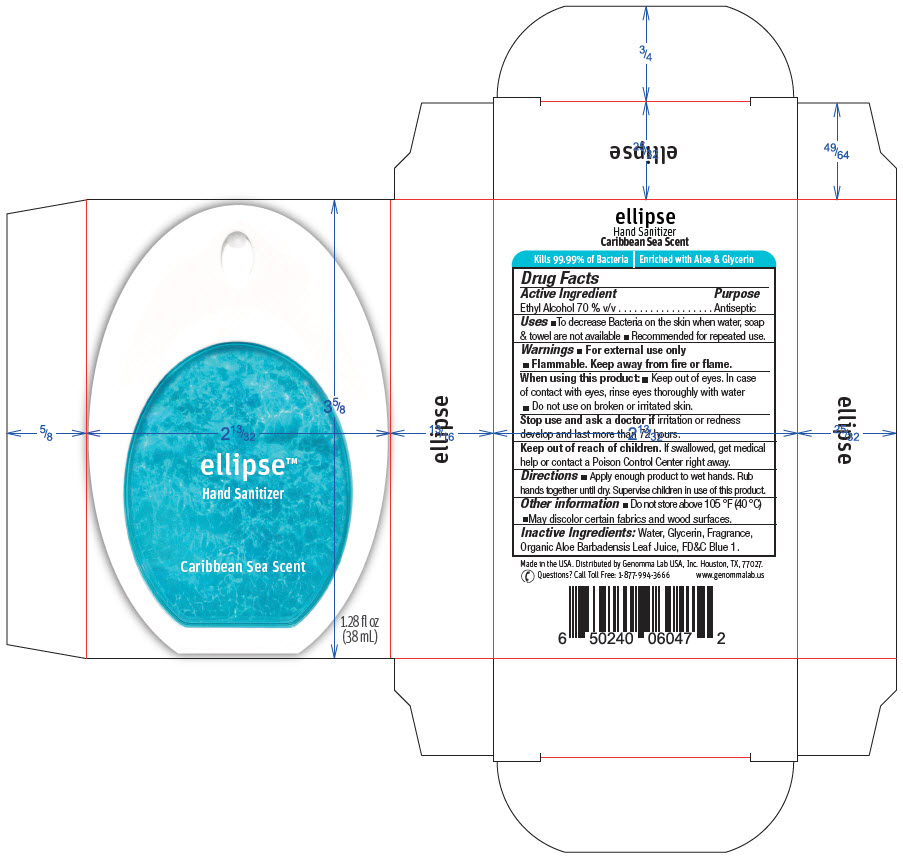
Ellipse™ Hand Sanitizer Caribbean Scent
Ellipse Hand Sanitizer by
Drug Labeling and Warnings
Ellipse Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Genomma Lab USA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ELLIPSE HAND SANITIZER CARIBBEAN SCENT- alcohol liquid
Genomma Lab USA
----------
Ellipse™ Hand Sanitizer
Caribbean Scent
Uses
- To decrease Bacteria on the skin when water, soap & towel are not available
- Recommended for repeated use.
Warnings
- For external use only
- Flammable. Keep away from fire or flame.
| ELLIPSE HAND SANITIZER
CARIBBEAN SCENT
alcohol liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genomma Lab USA (832323534) |
Revised: 1/2025
Document Id: d57f520a-2213-40a2-8fb0-3deb15056c39
Set id: ddb6a242-f878-4b8f-9c19-ab6a3a2260ae
Version: 2
Effective Time: 20250129
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.