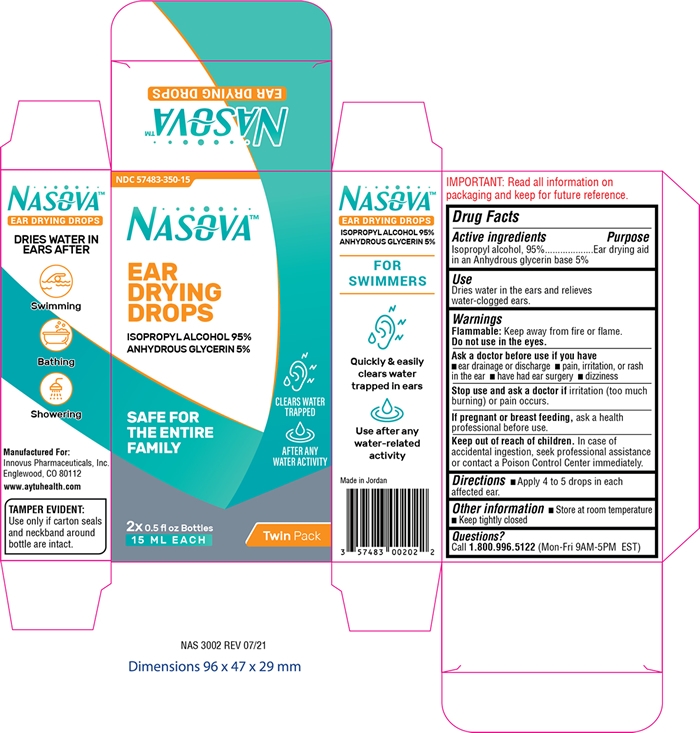
Nasova Ear Drying Drops by Innovus Pharmaceuticals, Inc. / AMMAN PHARMACEUTICAL INDUSTRIES Nasova Ear Drying Drops
Nasova Ear Drying Drops by
Drug Labeling and Warnings
Nasova Ear Drying Drops by is a Otc medication manufactured, distributed, or labeled by Innovus Pharmaceuticals, Inc., AMMAN PHARMACEUTICAL INDUSTRIES. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NASOVA EAR DRYING DROPS- isopropyl alcohol 95% solution/ drops
Innovus Pharmaceuticals, Inc.
----------
Nasova Ear Drying Drops
Aslk a doctor before use if you have
- ear drainage or discharge
- pain, irritation, or rash in the ear
- have had ear surgery
- dizziness
| NASOVA EAR DRYING DROPS
isopropyl alcohol 95% solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Innovus Pharmaceuticals, Inc. (962507187) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AMMAN PHARMACEUTICAL INDUSTRIES | 534677849 | manufacture(57483-350) | |
Revised: 7/2024
Document Id: 7bd10ae1-0327-4bb4-bb97-785436066745
Set id: ddb9359e-2fc4-4ce3-a7d2-027b17f01161
Version: 4
Effective Time: 20240720