E MEI SHAN MEDICATED- camphor and menthol plaster
E MEI SHAN MEDICATED by
Drug Labeling and Warnings
E MEI SHAN MEDICATED by is a Otc medication manufactured, distributed, or labeled by MADISON ONE ACME INC, Zhejiang Dingtai Pharmaceutical Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR/PHARMACIST
-
WHEN USING
When using this product
■ avoid contact with the eyes or mucous membranes
■ do not bandage tightly
■ do not apply heat to the area in the form of heating pads, hot water bottles, or lamps (doing so increases the risk of serious burns)
■ avoid placing on extremely hairy areas of skin to avoid irritation upon removing the plaster -
STOP USE
Stop use and ask a doctor if
■ condition worsens
■ symptoms persist for more than 7 days
■ symptoms clear up and occur again within a few days
■ excessive irritation of the skin develops
■ nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
■ you feel actual pain or experience blistering or burning after application (it is normal to feel a warming or cooling sensation)
■ when using for pain of arthritis:
■ pain persists for more than 10 days
■ redness is present
■ in conditions affecting children under 12 years of age - ADVERSE REACTIONS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
■ adults and children 2 years of age and older
■ clean and dry affected area
■ remove the protective film from the plaster
■ apply plaster to affected area not more than 3 to 4 times daily
■ remove plaster from the skin after at most 8-hour application
■ wet plaster with warm water before removing from the skin
■ children under 2 years of age: consult your physician - STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients
Angelica sinensis root, ocimum gratissimum leaf oil, butylated hydroxytoluene, cinnamon oil, saposhnikovia divaricata root, angelica dahurica root, ginger, lanolin, natural latex rubber, petrolatum, rosin, safflower, nepeta tenuifolia flowering top, and zinc oxide on an elastic cloth and covered with a protective paper film. - QUESTIONS
-
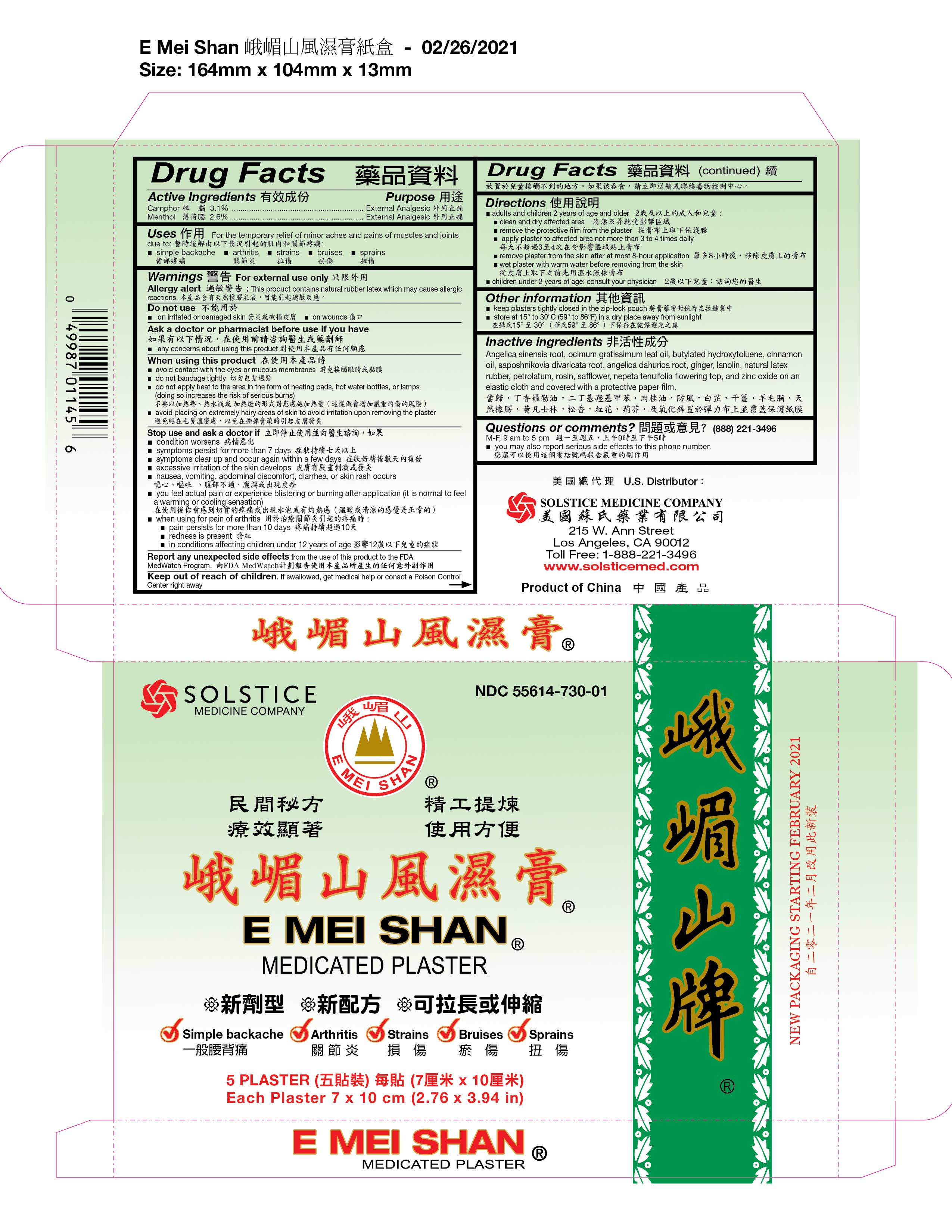
PRINCIPAL DISPLAY PANEL
E MEI SHAN MEDICATED PLASTER
NDC: 55614-730-01
Simple backache, Arthritis, Strains, Bruises, Sprains
5 Plaster
Each Plaster 7 x 10 cm (2.76 x 3.94 in)
-
INGREDIENTS AND APPEARANCE
E MEI SHAN MEDICATED
camphor and menthol plasterProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55614-730 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 31 mg MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 26 mg Inactive Ingredients Ingredient Name Strength ANGELICA SINENSIS ROOT (UNII: B66F4574UG) OCIMUM GRATISSIMUM LEAF OIL (UNII: AVG2506KDV) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CINNAMON OIL (UNII: E5GY4I6YCZ) SAPOSHNIKOVIA DIVARICATA ROOT (UNII: 8H84LFK2QD) ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) GINGER (UNII: C5529G5JPQ) LANOLIN (UNII: 7EV65EAW6H) NATURAL LATEX RUBBER (UNII: 2LQ0UUW8IN) PETROLATUM (UNII: 4T6H12BN9U) ROSIN (UNII: 88S87KL877) SAFFLOWER (UNII: 4VBL71TY4Y) NEPETA TENUIFOLIA FLOWERING TOP (UNII: 2FN3BA1MZE) ZINC OXIDE (UNII: SOI2LOH54Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55614-730-01 1 in 1 BOX 02/16/2021 1 5 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/16/2021 Labeler - MADISON ONE ACME INC (096196758) Registrant - MADISON ONE ACME INC (096196758) Establishment Name Address ID/FEI Business Operations Zhejiang Dingtai Pharmaceutical Co., Ltd 420598724 manufacture(55614-730)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.