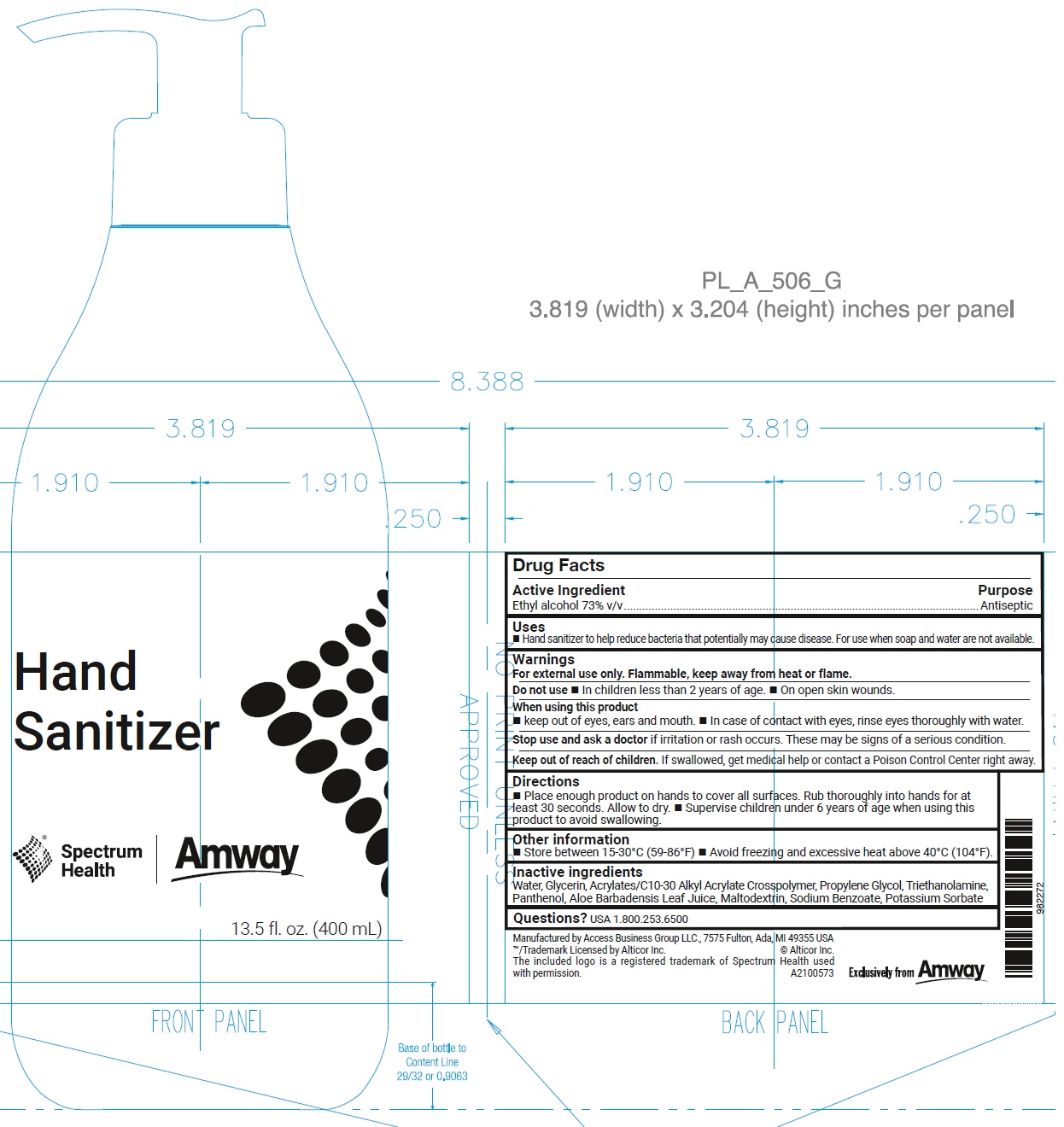
Spectrum Health - Amway Hand Sanitizer
Spectrum Health - Amway Hand Sanitizer by
Drug Labeling and Warnings
Spectrum Health - Amway Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Access Business Group LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SPECTRUM HEALTH - AMWAY HAND SANITIZER- alcohol gel
Access Business Group LLC
----------
Spectrum Health - Amway Hand Sanitizer
Uses
- Hand sanitizer to help reduce bacteria that potentially may cause disease. For use when soap and water are not available.
Warnings
For external use only. Flammable, keep away from heat or flame.
Directions
- Place enough product on hands to cover all surfaces. Rub thoroughly into hands for at least 30 seconds. Allow to dry.
- Supervise children under 6 years of age when using this product to avoid swallowing.
Other information
- Store between 15-30°C (59-86°F)
- Avoid freezing and excessive heat above 40°C (104°F).
| SPECTRUM HEALTH - AMWAY HAND SANITIZER
alcohol gel |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Access Business Group LLC (839830713) |
Revised: 9/2024
Document Id: 21c44a94-9928-9d5a-e063-6394a90a7af0
Set id: ddd39260-f69e-4c29-9f46-125fc4bf4a84
Version: 3
Effective Time: 20240910