Comirnaty Vaccine
Comirnaty by
Drug Labeling and Warnings
Comirnaty by is a Other medication manufactured, distributed, or labeled by Pfizer Laboratories Div Pfizer Inc, Pfizer Inc, Pfizer Manufacturing Belgium NV, Pharmacia & Upjohn Company LLC, Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC, BioNTech Manufacturing GmbH, BioNTech Manufacturing Marburg GmbH, Pfizer Ireland Pharmaceuticals, Labor LS SE & Co. KG, BioNTech Innovative Manufacturing Services GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
COMIRNATY- covid-19 vaccine, mrna injection, suspension
Pfizer Laboratories Div Pfizer Inc
----------
Comirnaty Vaccine
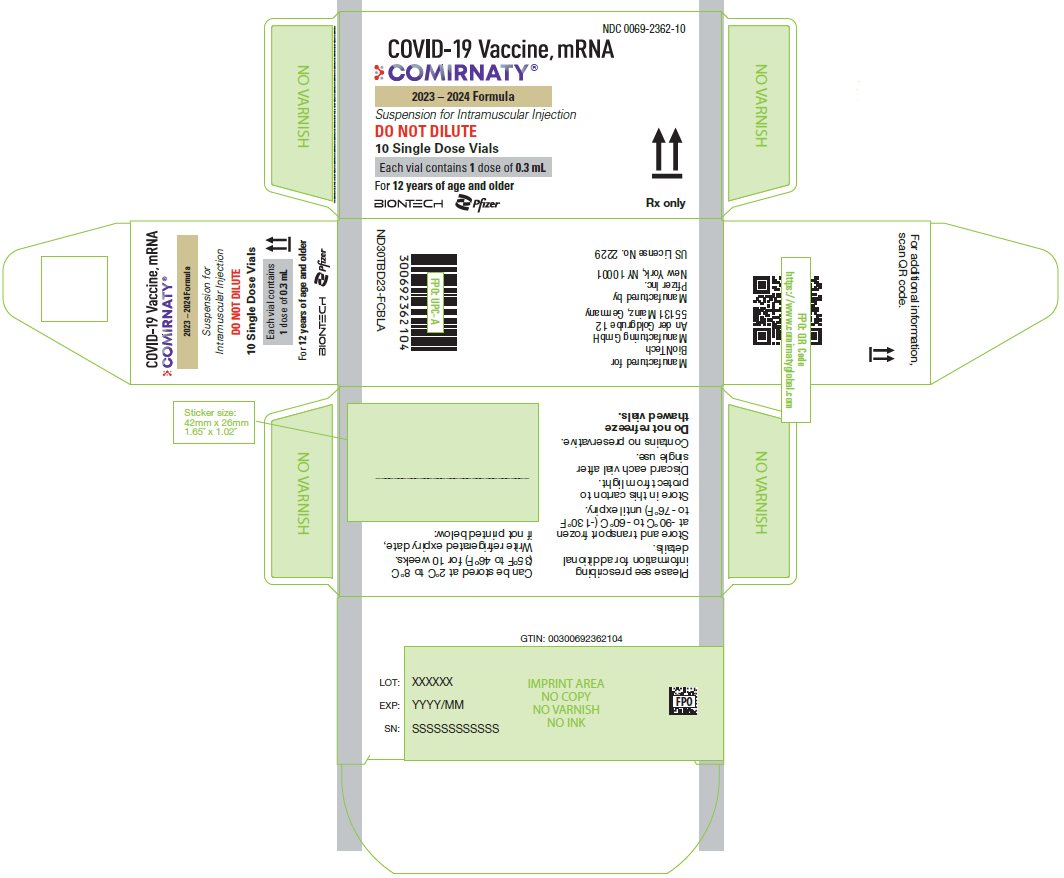
PRINCIPAL DISPLAY PANEL – 0.3 mL Single Dose Vial Label
COVID-19 Vaccine, mRNA
COMIRNATY ®
Rx only
2023 – 2024 Formula
DO NOT DILUTE
Age 12y & older
Vial contains 1 dose of 0.3 mL
For intramuscular use.
US License No. 2229
BioNTech Manufacturing GmbH & Pfizer Inc.
NDC: 0069-2362-01
PRINCIPAL DISPLAY PANEL – 10 Single Dose Vial Carton
NDC: 0069-2362-10
COVID-19 Vaccine, mRNA
COMIRNATY ®
2023 – 2024 Formula
Suspension for Intramuscular Injection
DO NOT DILUTE
10 Single Dose Vials
Each vial contains 1 dose of 0.3 mL
For 12 years of age and older
BIONTECH
Pfizer
Rx only
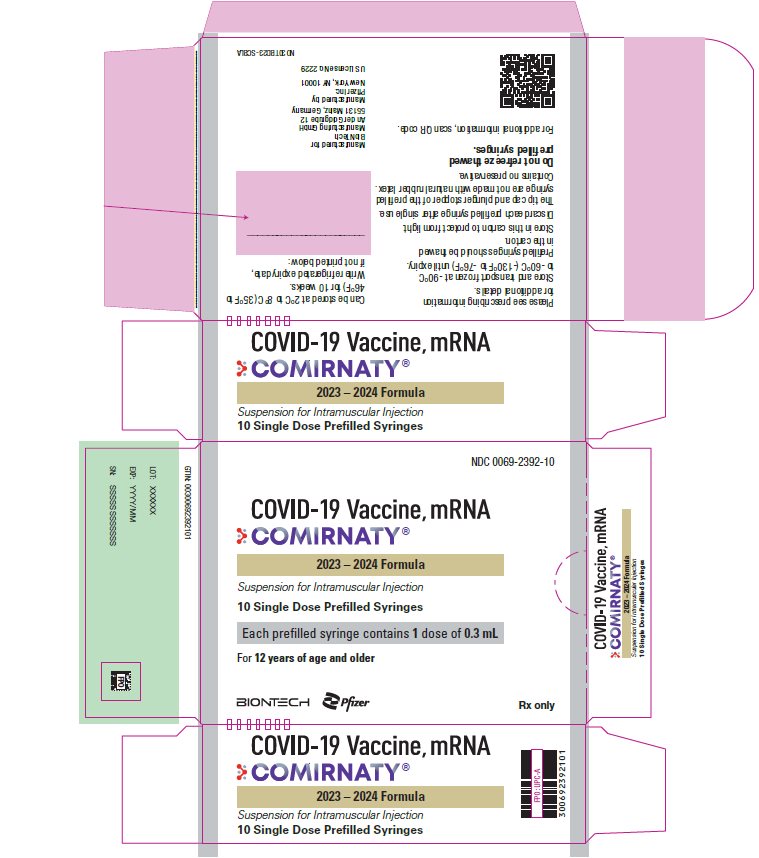
PRINCIPAL DISPLAY PANEL – 0.3 mL Prefilled Syringe Label
NDC: 0069-2392-01
LOT:/EXP:
XXXXXXXX
FPO
LOT:/EXP:
COVID-19 Vaccine, mRNA
COMIRNATY®
YYYY/MM/DD
Age 12y & older
Contains 1 dose
of 0.3 mL
For intramuscular use.
US License No. 2229
BioNTech Manufacturing
GmbH & Pfizer Inc.
NDC: 0069-2392-01
Rx only
COVID-19
Vaccine, mRNA
COMIRNATY®
2023 – 2024 Formula
PRINCIPAL DISPLAY PANEL – 10 Prefilled Syringe Carton
NDC: 0069-2392-10
COVID-19 Vaccine, mRNA
COMIRNATY ®
2023 – 2024 Formula
Suspension for Intramuscular Injection
10 Single Dose Prefilled Syringes
Each prefilled syringe contains 1 dose of 0.3 mL
For 12 years of age and older
BIONTECH
Pfizer
Rx only
| COMIRNATY
covid-19 vaccine, mrna injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| COMIRNATY
covid-19 vaccine, mrna injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) |
| Registrant - Pfizer Inc (113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Manufacturing Belgium NV | 370156507 | PACK(0069-2362, 0069-2392) , MANUFACTURE(0069-2362, 0069-2392) , ANALYSIS(0069-2362, 0069-2392) , LABEL(0069-2362, 0069-2392) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | MANUFACTURE(0069-2362) , LABEL(0069-2362) , PACK(0069-2362) , ANALYSIS(0069-2362) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC | 174350868 | ANALYSIS(0069-2362, 0069-2392) , API MANUFACTURE(0069-2362, 0069-2392) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Manufacturing GmbH | 314382536 | ANALYSIS(0069-2362, 0069-2392) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Manufacturing Marburg GmbH | 313270335 | ANALYSIS(0069-2362, 0069-2392) , API MANUFACTURE(0069-2362, 0069-2392) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Ireland Pharmaceuticals | 985586408 | ANALYSIS(0069-2362, 0069-2392) , API MANUFACTURE(0069-2362, 0069-2392) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Labor LS SE & Co. KG | 314929072 | ANALYSIS(0069-2362, 0069-2392) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioNTech Innovative Manufacturing Services GmbH | 537365801 | ANALYSIS(0069-2362, 0069-2392) | |
Trademark Results [Comirnaty]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COMIRNATY 88942267 not registered Live/Pending |
BioNTech SE 2020-06-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.