SUTAB- sodium sulfate, magnesium sulfate, and potassium chloride tablet
SUTAB by
Drug Labeling and Warnings
SUTAB by is a Prescription medication manufactured, distributed, or labeled by Braintree Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use
SUTAB ®safely and effectively. See full prescribing information for
SUTAB.
SUTAB (sodium sulfate, magnesium sulfate, and potassium chloride)
tablets, for oral use
Initial U.S. Approval: 2020RECENT MAJOR CHANGES
INDICATIONS AND USAGE
SUTAB is an osmotic laxative indicated for cleansing of the colon in preparation for colonoscopy in adults. (1)
DOSAGE AND ADMINISTRATION
For complete information on preparation before colonoscopy and administration of the dosage regimen, see full prescribing information. ( 2.1, 2.2)
Preparation and Administration ( 2.1)
- Administration of two doses (24 tablets) are required for a complete preparation for colonoscopy.
- SUTAB is supplied as two bottles each containing 12 tablets. Twelve (12) tablets are equivalent to one dose.
- Each SUTAB bottle contains a desiccant. Remove and discard the desiccantfrom both bottles the evening prior to the colonoscopy.
- Must consume water with each dose and an additional 32 ounces of water after each dose.
- Do not take other laxatives.
- Administer oral medications at least 1 hour before starting each dose of SUTAB.
- If taking tetracycline or fluoroquinolone antibiotics, iron, digoxin, chlorpromazine, or penicillamine, take these medications at least 2 hours before and not less than 6 hours after administration of each dose.
Recommended Split Dose (2-Day) Regimen ( 2.2)
Day 1, Dose 1: On the Evening Prior to Colonoscopy
- Open 1 bottle of 12 tablets. Remove and discard the desiccant. Remove and discard the desiccantfrom the second bottle and close the bottle. Use the second bottle for the second dose on the morning of the colonoscopy.
- Fill the provided container with 16 ounces of water (up to the fill line). Swallow each tablet with a sip of water and drink the entire amount over 15 to 20 minutes.
- Approximately one hour after the last tablet is ingested, fill the provided container a second time with 16 ounces of water (up to the fill line) and drink the entire amount over 30 minutes.
- Approximately 30 minutes after finishing the second container of water, fill the provided container with 16 ounces of water (up to the fill line) and drink the entire amount over 30 minutes.
Day 2, Dose 2: Morning of the Colonoscopy (5 to 8 hours prior to the colonoscopy and no sooner than 4 hours from starting Dose 1):
- Continue to consume only clear liquids until after the colonoscopy.
- Repeat Step 2 to Step 4 from Day 1, Dose 1.
- If patients experience preparation-related symptoms (e.g., nausea, bloating, cramping), pause or slow the rate of drinking the additional water until symptoms diminish.
- Complete all SUTAB tablets and water at least two hours prior to colonoscopy.
DOSAGE FORMS AND STRENGTHS
Tablets: 1.479 g sodium sulfate, 0.225 g magnesium sulfate, and 0.188 g potassium chloride. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Risk of fluid and electrolyte abnormalities: Encourage adequate hydration, assess concurrent medications and consider laboratory assessments prior to and after each use. ( 5.1, 7.1)
- Cardiac arrhythmias: Consider pre-dose and post-colonoscopy ECGs in patients at increased risk. ( 5.2)
- Seizures: Use caution in patients with a history of seizures and patients at increased risk of seizures, including medications that lower the seizure threshold. ( 5.3, 7.1)
- Patients with renal impairment or taking concomitant medications that affect renal function: Use caution, ensure adequate hydration and consider laboratory testing. ( 5.4, 7.1)
- Colonic Mucosal ulcerations: Consider potential for mucosal ulcerations when interpreting colonoscopy findings in patients with known or suspected inflammatory bowel disease. ( 5.5)
- Suspected GI obstruction or perforation: Rule out the diagnosis before administration. ( 4, 5.6)
- Hypersensitivity reactions, including anaphylaxis: Inform patients to seek immediate medical care if symptoms occur. ( 5.7)
- Risk of Gastrointestinal Complications with Ingestion of Desiccant: Postmarketing reports of ingestion of the desiccant along with SUTAB tablets has been reported and may be associated with risk of gastrointestinal complications and/or choking. ( 2.2, 5.8)
ADVERSE REACTIONS
Most common gastrointestinal adverse reactions are nausea, abdominal distension, vomiting and upper abdominal pain. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Braintree Laboratories, Inc. at 1-800-874-6756 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.DRUG INTERACTIONS
Drugs that increase risk of fluid and electrolyte imbalance ( 7.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
2.2 Recommended Split-Dose (2-Day) Dosage Regimen
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Fluid and Electrolyte Abnormalities
5.2 Cardiac Arrhythmias
5.3 Seizures
5.4 Use in Patients with Risk of Renal Injury
5.5 Colonic Mucosal Ulcerations and Ischemic Colitis
5.6 Use in Patients with Significant Gastrointestinal Disease
5.7 Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs That May Increase Risks of Fluid and Electrolyte Abnormalities
7.2 Potential for Reduced Drug Absorption
7.3 Stimulant Laxatives
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
- Correct fluid and electrolyte abnormalities before treatment with SUTAB [see Warnings and Precautions ( 5.1)]
- Administration of two doses of SUTAB (24 tablets) are required for a complete preparation for colonoscopy.
- SUTAB is supplied as two bottles each containing 12 tablets. Twelve (12) tablets are equivalent to one dose.
- Each SUTAB bottle contains a desiccant. Remove and discard the desiccantfrom both bottles of SUTAB the evening prior to the colonoscopy [see Dosage and Administration ( 2.2)] .
- Must consume water with each dose of SUTAB and an additional 32 ounces of water must be consumed after each dose [see Dosage and Administration (2.2) and Warnings and Precautions ( 5.1)] .
- Consume a low residue breakfast on the day before colonoscopy, followed by clear liquids up to 2 hours prior to colonoscopy.
- Do not drink milk or eat or drink anything colored red or purple.
- Do not drink alcohol.
- Do not take other laxatives while taking SUTAB.
- Administer oral medications at least 1 hour before starting each dose of SUTAB.
- If taking tetracycline or fluoroquinolone antibiotics, iron, digoxin, chlorpromazine, or penicillamine, take these medications at least 2 hours before and not less than 6 hours after administration of each dose of SUTAB.
- Stop consumption of all fluids at least 2 hours prior to the colonoscopy.
2.2 Recommended Split-Dose (2-Day) Dosage Regimen
The recommended Split-Dose (2-day) dosage regimen for adults consists of two doses of SUTAB: the first dose during the evening prior to colonoscopy and the second dose the next day, during the morning of the colonoscopy.
Instruct patients:
On the Day Prior to Colonoscopy:
- A low residue breakfast may be consumed. Examples of low residue foods are eggs, white bread, cottage cheese, yogurt, grits, coffee, tea.
- After breakfast, only clear liquids may be consumed until after the colonoscopy. Examples of clear liquids are coffee or tea (no cream or non-dairy creamer), fruit juices (without pulp), gelatin desserts (no fruit or topping), water, chicken broth, clear soda (such as ginger ale).
Day 1, Dose 1 - On the Evening Prior to Colonoscopy:
- Early in the evening prior to colonoscopy, open one bottle of 12 tablets. Remove and discard the desiccant.Remove and discard the desiccant from the second bottle and close the bottle. Use the second bottle for the second dose on the morning of the colonoscopy.
- Fill the provided container with 16 ounces of water (up to the fill line). Swallow one tablet at a time with a sip of water. Finish taking the 12 tablets and drinking the entire amount of water within 15 to 20 minutes.
- Approximately one hour after the last tablet is ingested, fill the provided container a second time with 16 ounces of water (up to the fill line) and drink the entire amount over 30 minutes.
- Approximately 30 minutes after finishing the second container of water, fill the provided container again with 16 ounces of water (up to the fill line) and drink the entire amount over 30 minutes.
If patients experience preparation-related symptoms (e.g. nausea, bloating, cramping), pause or slow the rate of drinking the additional water until symptoms diminish.
Day 2, Dose 2 – The Morning of the Colonoscopy(5 to 8 hours prior to the colonoscopy and no sooner than 4 hours from starting Dose 1):
- Continue to consume only clear liquids until after the colonoscopy.
- Repeat Step 2 to Step 4 from Day 1, Dose 1.
- If patients experience preparation-related symptoms (e.g., nausea, bloating, cramping), pause or slow the rate of drinking the additional water until symptoms diminish.
- Complete taking all SUTAB tablets and water at least two hours prior to colonoscopy.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Fluid and Electrolyte Abnormalities
Advise all patients to hydrate adequately before, during, and after the use of SUTAB. If a patient develops significant vomiting or signs of dehydration after taking SUTAB, consider performing postcolonoscopy lab tests (electrolytes, creatinine, and BUN). Fluid and electrolyte disturbances can lead to serious adverse events including cardiac arrhythmias, seizures and renal impairment. Correct fluid and electrolyte abnormalities before treatment with SUTAB. Use SUTAB with caution in patients with conditions, or who are using medications, that increase the risk for fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and renal impairment [see Drug Interactions ( 7.1)] .
5.2 Cardiac Arrhythmias
There have been rare reports of serious arrhythmias associated with the use of ionic osmotic laxative products for bowel preparation. Use caution when prescribing SUTAB for patients at increased risk of arrhythmias (e.g., patients with a history of prolonged QT, uncontrolled arrhythmias, recent myocardial infarction, unstable angina, congestive heart failure, or cardiomyopathy). Consider predose and post-colonoscopy ECGs in patients at increased risk of serious cardiac arrhythmias.
5.3 Seizures
There have been reports of generalized tonic-clonic seizures and/or loss of consciousness associated with use of bowel preparation products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities.
Use caution when prescribing SUTAB for patients with a history of seizures and in patients at increased risk of seizure, such as patients taking medications that lower the seizure threshold (e.g., tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia [see Drug Interactions ( 7.1)] .
5.4 Use in Patients with Risk of Renal Injury
Use SUTAB with caution in patients with impaired renal function or patients taking concomitant medications that may affect renal function (such as diuretics, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or non-steroidal anti-inflammatory drugs) [see Drug Interactions ( 7.1)] . These patients may be at risk for renal injury. Advise these patients of the importance of adequate hydration with SUTAB and consider performing baseline and postcolonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients [see Use in Specific Populations ( 8.6)] .
5.5 Colonic Mucosal Ulcerations and Ischemic Colitis
Osmotic laxative products may produce colonic mucosal aphthous ulcerations, and there have been reports of more serious cases of ischemic colitis requiring hospitalization. Concurrent use of stimulant laxatives and SUTAB may increase these risks [see Drug Interactions ( 7.3)] . Consider the potential for mucosal ulcerations resulting from the bowel preparation when interpreting colonoscopy findings in patients with known or suspect inflammatory bowel disease (IBD).
5.6 Use in Patients with Significant Gastrointestinal Disease
If gastrointestinal obstruction or perforation is suspected, perform appropriate diagnostic studies to rule out these conditions before administering SUTAB [see Contraindications ( 4)] .
Use in caution in patients with severe active ulcerative colitis.
5.7 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, angioedema, dyspnea, rash, pruritis and urticaria have been reported with SUTAB [see Adverse Reactions ( 6.2)] . Inform patients of the signs and symptoms of anaphylaxis and instruct them to seek immediate medical care should signs and symptoms occur.
-
6 ADVERSE REACTIONS
The following serious or otherwise important adverse reactions for bowel preparations are described elsewhere in the labeling:
- Serious Fluid and Electrolyte Abnormalities [see Warnings and Precautions ( 5.1)]
- Cardiac Arrhythmias [see Warnings and Precautions ( 5.2)]
- Seizures [see Warnings and Precautions ( 5.3)]
- Patients with Risk of Renal Injury [see Warnings and Precautions ( 5.4)]
- Colonic Mucosal Ulceration and Ischemic Colitis [see Warnings and Precautions ( 5.5)]
- Patients with Significant Gastrointestinal Disease [see Warnings and Precautions ( 5.6)]
- Hypersensitivity Reactions [see Warnings and Precautions ( 5.7)]
- Risk of Gastrointestinal Complications with Ingestion of Desiccant [see Warnings and Precautions ( 5.8)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in clinical studies of another drug and may not reflect the rates observed in practice.
The safety of SUTAB was evaluated in two randomized, parallel group, multicenter, investigator blinded clinical trials in 941 adult patients undergoing colonoscopy. The active comparators were polyethylene glycol 3350, sodium sulfate, sodium chloride, potassium chloride, ascorbic acid and sodium ascorbate for oral solution in Study 1 and sodium picosulfate, magnesium oxide, and anhydrous citric acid for oral solution in Study 2 [see Clinical Studies ( 14)] .
Adverse Gastrointestinal Reactions Reported by Symptom Questionnaire
In Studies 1 and 2, patients were queried for selected gastrointestinal adverse reactions of stomach cramping (upper abdominal pain), stomach bloating (abdominal distention), nausea and vomiting using a standard questionnaire following completion of study drug and prior to colonoscopy on the day of colonoscopy. Patients reporting selected gastrointestinal symptom(s) rated the intensity as mild, moderate or severe.A total of 52% (287/552) of patients in Study 1 and 52% (202/389) in Study 2 reported at least one selected gastrointestinal adverse reaction when queried using the standard questionnaire. Tables 1 and 2 show results for each gastrointestinal adverse reaction reported by patients using the standard questionnaire, including severity.
Table 1: Gastrointestinal Symptoms by Severity aFrom Symptom Questionnaire in Adult Patients Following Colon Cleansing and Prior to Colonoscopy – Study 1 b a Mild: barely noticeable, does not influence functioning causing no limitations of usual activities;
Moderate: makes participant uncomfortable, influences functioning causing some limitations of usual activities;
Severe: severe discomfort, treatment needed, severe and undesirable, causing inability to carry out usual activitiesbStudy 1 was not designed to support comparative claims for SUTAB for the adverse reactions reported in this table.
cPercentage represents n/N for patients who experienced each gastrointestinal adverse reaction on the symptom questionnaire based on the total number of patients per treatment arm.
Symptom SUTAB
Polyethylene glycol 3350, sodium sulfate, sodium chloride, potassium chloride, ascorbic acid and sodium ascorbate
Total Number or Patients per Treatment Arm (N) 281 271 Patients with at Least One Gastrointestinal Adverse Reaction from Symptom Questionnaire 163 124 % Nausea c 48 26 Mild 71 77 Moderate 27 23 Severe 2 0 % Abdominal Distension c,d 29 22 Mild 68 71 Moderate 30 29 Severe 1 0 % Vomiting c 23 5 Mild 48 46 Moderate 52 54 Severe 0 0 % Upper Abdominal Pain c 16 18 Mild 65 71 Moderate 35 29 Severe 0 0 Total Number of Patients per
Treatment Arm (N)281 271 Table 2: Gastrointestinal Symptoms by Severity aFrom Symptom Questionnaire in Adult Patients Following Colon Cleansing and Prior to Colonoscopy – Study 2 b a Mild: barely noticeable, does not influence functioning causing no limitations of usual activities;
Moderate: makes participant uncomfortable, influences functioning causing some limitations of usual activities;
Severe: severe discomfort, treatment needed, severe and undesirable, causing inability to carry out usual activitiesbStudy 2 was not designed to support comparative claims for SUTAB for the adverse reactions reported in this table.
cPercentage represents n/N for patients who experienced each gastrointestinal adverse reaction on the symptom questionnaire based on the total number of patients per treatment arm.
Symptom SUTAB
Sodium picosulfate, magnesium oxide, and anhydrous citric acid
Total Number or Patients per Treatment Arm (N) 190 199 Patients with at Least One Gastrointestinal Adverse Reaction from Symptom Questionnaire 135 67 % Nausea c 52 18 Mild 74 94 Moderate 20 6 Severe 6 0 % Abdominal Distension c 34 15 Mild 73 69 Moderate 27 31 Severe 0 0 % Vomiting c 16 2 Mild 53 33 Moderate 47 67 Severe 0 0 % Upper Abdominal Pain c 23 13 Mild 82 100 Moderate 16 0 Severe 2 0 Total Number of Patients per
Treatment Arm (N)190 199 Patients with at Least One
Gastrointestinal Adverse Reaction
from Symptom Questionnaire135 67 Additional Adverse Reactions Reported in Studies 1 and 2
In addition to the gastrointestinal symptoms reported on the standard questionnaire (Tables 1 and 2), other adverse reactions reported in at least 2% of patients in either treatment arm in Studies 1 and 2 were: dizziness in Study 1 (0% SUTAB and 2% comparator); and hypermagnesemia (2% SUTAB and 2% comparator) and increased liver function test (including ALT, AST and bilirubin) (3% SUTAB and 1% comparator) in Study 2.Laboratory Changes
Electrolyte Abnormalities
Shifts in serum electrolytes from normal at baseline to above the upper end of normal following study drug on the day of colonoscopy in at least 2% of patients in either treatment arm and at least 2% greater in patients treated with SUTAB than treated with comparator in either Study 1 or Study 2 were: magnesium (27% SUTAB and 5% comparator in Study 1), and serum osmolality (44% SUTAB and 28% comparator in Study 2). These changes were transient and resolved without intervention.Renal Function Parameters
Decreases in creatinine clearance and increases in blood urea nitrogen (BUN) were reported in less than 1% of patients in both SUTAB and comparator arms in both trials.6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of SUTAB. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal: gastric ulceration, gastritis
Hypersensitivity: anaphylaxis, angioedema, dyspnea, rash, pruritus, urticaria [see Warnings and Precautions ( 5.7)]
-
7 DRUG INTERACTIONS
7.1 Drugs That May Increase Risks of Fluid and Electrolyte Abnormalities
Use caution when prescribing SUTAB to patients taking medications that increase the risk of fluid and electrolyte disturbances or may increase the risk of adverse events of seizure, arrhythmias, and prolonged QT in the setting of fluid and electrolyte abnormalities [see Warnings and Precautions ( 5.1, 5.2, 5.3, 5.4)] .
7.2 Potential for Reduced Drug Absorption
SUTAB can reduce the absorption of other co-administered drugs [see Dosage and Administration ( 2.1)] :
- Administer oral medications at least one hour before starting each dose of SUTAB.
- Administer tetracycline and fluoroquinolone antibiotics, iron, digoxin, chlorpromazine, and penicillamine at least 2 hours before and not less than 6 hours after administration of each dose of SUTAB to avoid chelation with magnesium.
7.3 Stimulant Laxatives
Concurrent use of stimulant laxatives and SUTAB may increase the risk of mucosal ulceration or ischemic colitis. Avoid use of stimulant laxatives (e.g., bisacodyl, sodium picosulfate) while taking SUTAB [see Warnings and Precautions ( 5.5)] .
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on SUTAB use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. No reproduction or developmental studies in animals have been conducted with sodium sulfate, magnesium sulfate, and potassium chloride (SUTAB).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no available data on the presence of SUTAB in human or animal milk, the effects of on the breastfed child, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SUTAB and any potential adverse effects on the breastfed child from SUTAB or from the underlying maternal condition.
8.5 Geriatric Use
Of the 471 patients who received SUTAB in pivotal clinical trials, 150 (32%) were 65 years of age or older, and 25 (5%) were 75 years of age or older. No differences in safety or effectiveness of SUTAB were observed between geriatric patients and younger patients. Elderly patients are more likely to have decreased hepatic, renal or cardiac function and may be more susceptible to adverse reactions resulting from fluid and electrolyte abnormalities [see Warnings and Precautions ( 5.1)] .
8.6 Renal Impairment
Use SUTAB with caution in patients with renal impairment or patients taking concomitant medications that may affect renal function. These patients may be at risk for renal injury. Advise these patients of the importance of adequate hydration before, during and after use of SUTAB and consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients [see Warning and Precautions ( 5.4)] .
-
10 OVERDOSAGE
Overdosage of more than the recommended dose of SUTAB may lead to severe electrolyte disturbances, as well as dehydration and hypovolemia, with signs and symptoms of these disturbances [see Warnings and Precautions ( 5.1, 5.2, 5.3)] . Monitor for fluid and electrolyte disturbances and treat symptomatically.
-
11 DESCRIPTION
SUTAB (sodium sulfate, magnesium sulfate, and potassium chloride) tablets is an orally administered osmotic laxative and is provided as two bottles, each containing 12 tablets. Each tablet contains: 1.479 g sodium sulfate, 0.225 g magnesium sulfate, and 0.188 g potassium chloride. Inactive ingredients include: polyethylene glycol 8000, sodium caprylate, and ethylene glycol and vinyl alcohol graft copolymer.
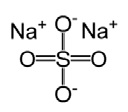
Sodium Sulfate, USP
The molecular formula is Na 2SO 4. The average molecular weight is 142.04. The structural formula is:
Magnesium Sulfate, USP
The molecular formula is MgSO 4. The average molecular weight is 120.37. The structural formula is:

Potassium Chloride, USP
The molecular formula is KCl. The average molecular weight is 74.55. The structural formula is:

-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The primary mode of action is osmotic action of sodium sulfate and magnesium sulfate, which induce a laxative effect. The physiological consequence is increased water retention in the lumen of the colon, resulting in loose stools.
12.3 Pharmacokinetics
Absorption
After the oral administration of SUTAB to patients in clinical studies, the median serum sulfate concentration increased by about 2.5-fold at 5 to 8 hours post Dose 2 (0.61 mmol/L) compared to baseline (0.25 mmol/L) and returned to baseline by 24 to 48 hours after colonoscopy.Elimination
Fecal excretion is the primary route of sulfate elimination.Use in Specific Populations
Patients with Renal Impairment
The disposition of sulfate after ingestion of a sulfate-based product containing sodium sulfate, potassium sulfate, and magnesium sulfate similar to SUTAB was studied in patients (N=6) with moderate renal impairment (creatinine clearance of 30 to 49 mL/min). In patients with moderate renal impairment, mean AUC was 54% higher and mean Cmax was 44% higher than healthy subjects. The mean sulfate concentrations in healthy subjects and in patients with moderate renal impairment returned to their respective baselines by Day 6 after dose initiation. Urinary excretion of sulfate over 30 hours after the first dose was approximately 16% lower in patients with moderate renal impairment than in healthy subjects. These differences are not considered clinically meaningful.Patients with Hepatic Impairment
The disposition of sulfate after ingestion of a sulfate-based product containing sodium sulfate, potassium sulfate, and magnesium sulfate similar to SUTAB was also studied in patients (N=6) with mild-moderate hepatic impairment (Child-Pugh grades A and B). Systemic exposure of serum sulfate (AUC and Cmax) was similar between healthy subjects and patients with hepatic impairment. The mean sulfate concentrations in healthy subjects and in patients with mild to moderate hepatic impairment returned to their respective baselines by Day 6 after dose initiation. Urinary excretion of sulfate over 30 hours after the first dose was similar between patients with hepatic impairment and healthy subjects. -
13 NONCLINICAL TOXICOLOGY
13.2 Animal Toxicology and/or Pharmacology
Animal toxicology studies with sodium sulfate, magnesium sulfate, and potassium chloride (SUTAB) have not been conducted. Sulfate salts of sodium, potassium, and magnesium, were administered orally (gavage) to rats and dogs up to 28 days up to a maximum daily dose of 5 grams/kg/day (approximately 0.9 and 3 times for rats and dogs, respectively, the recommended SUTAB human dose of 45.4 grams/day or 0.86 grams/kg based on the body surface area). In rats, the sulfate salts caused diarrhea and electrolyte and metabolic changes, including hypochloremia, hypokalemia, hyponatremia, lower serum osmolality, and high serum bicarbonate. Significant renal changes included increased fractional sodium excretion, increased urinary sodium and potassium excretion, and alkaline urine in both male and females. In addition, creatinine clearance was significantly decreased in females at the highest dose. No microscopic renal changes were seen. In dogs, the sulfate salts caused emesis, excessive salivation, excessive drinking of water, and abnormal excreta (soft and/or mucoid feces and/or diarrhea) and increased urine pH and sodium excretion.
-
14 CLINICAL STUDIES
The colon cleansing efficacy of SUTAB was evaluated in two randomized, single-blind, active-controlled, multicenter trials (Study 1 and Study 2). These trials included adult subjects undergoing colonoscopy for colorectal cancer screening and surveillance, or diagnostic colonoscopy, including subjects with abdominal pain, diarrhea, constipation and non-severe inflammatory bowel disease.
In Study 1 (BLI4700-301; NCT 03404401), 548 adult patients were included in the efficacy analysis. Patients ranged in age from 19 to 84 years (median age 59 years) and 56% were female. Racial distribution was 78% Caucasian, 16% African-American, and 11% Hispanic or Latino. Patients were randomized to one of the following two colon preparation regimens: SUTAB or polyethylene glycol 3350, sodium sulfate, sodium chloride, potassium chloride, ascorbic acid and sodium ascorbate for oral solution. Both preparations were administered according to a split-dose regimen [see Dosage and Administration ( 2.2)] . Patients receiving SUTAB were limited to a low residue breakfast followed by clear liquids on the day prior to the day of colonoscopy; patients receiving the comparator bowel prep were allowed to have a normal breakfast and a light lunch, followed by clear liquids and/or yogurt for dinner. Approximately 97% of patients in the study completed both doses of preparation (98% of SUTAB patients and 95% of comparator patients).
In Study 2 (BLI4700-302; NCT 03261960), 388 adult patients were included in the efficacy analysis. Patients ranged in age from 23 to 83 years (median age 58 years) and 58% were female. Racial distribution was 94% Caucasian, 9% Hispanic or Latino, and 5% African-American. Patients were randomized to one of the following two colon preparation regimens: SUTAB or sodium picosulfate, magnesium oxide, and anhydrous citric acid for oral solution. Both preparations were administered according to a split-dose regimen [see Dosage and Administration ( 2.2)] . Patients receiving SUTAB were limited to a low residue breakfast followed by clear liquids on the day prior to the day of colonoscopy; patients receiving the comparator bowel prep were only allowed clear liquids on the day prior to colonoscopy. Approximately 98% of patients in the study completed both doses of preparation (98% of SUTAB patients and 99% of comparator patients).
The primary efficacy endpoint in each trial was the proportion of patients with successful colon cleansing, as assessed by the blinded colonoscopist utilizing the four-point scaled described below. Success was defined as an overall cleansing assessment of 3 (Good) or 4 (Excellent).
Score Grade Description 1 Poor Large amount of fecal residue, additional bowel preparation required. 2 Fair Enough feces even after washing and suctioning to prevent clear visualization of the entire colonic mucosa. 3 Good Feces and fluid requiring washing and suctioning, but still achieves clear visualization of the entire colonic mucosa. 4 Excellent No more than small bits of feces/fluid which can be suctioned easily; achieves clear visualization of the entire colonic mucosa. Results for the primary endpoint in Studies 1 and 2 are shown in Table 3. In both trials, SUTAB was non-inferior to the comparator.
Table 3 Proportion of Adult Patients with Overall Cleansing Success ain Two Controlled Trials with a Split-Dose Regimen aSuccess was defined as an overall cleaning assessment of 3 (Good) or 4 (Excellent) by the blinded endoscopist, scores were assigned on withdrawal of colonoscope.
btreatment differences and confidence intervals were adjusted by study sites based on Mantel-Haenszel method
ccomparator in Study 1 was polyethylene glycol 3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate and ascorbic acid for oral solution
dcomparator in Study 2 was sodium picosulfate, magnesium oxide, and anhydrous citric acid for oral solution
enon-inferior
SUTAB
% (n/N)
Comparator
% (n/N)
SUTAB-comparator
Difference b(%) 99% Confidence
Interval b
Study 1 92%
(257/278)
89% c
(241/270)
3.0 (-3.2, 9.3) e
Study 2 92% (175/190)
88% d (174/198)
3.1 (-4.5, 10.7) e -
16 HOW SUPPLIED/STORAGE AND HANDLING
Each tablet of SUTAB contains 1.479 g sodium sulfate, 0.225 g magnesium sulfate, and 0.188 g potassium chloride. The tablets are white to off-white, film coated, oblong, and biconvex with flat sides, debossed with S24 on one side.
Each carton of SUTAB (NDC: 52268-201-01) contains:
- Two bottles, each bottle (NDC: 52268-200-01) contains 12 tablets.
- One container with a 16-ounce fill line.
Note to Pharmacist: Inform the patient to removeand discardthe desiccant from both bottles of SUTAB the evening prior to the colonoscopy [see Dosage and Administration ( 2.2)]
Storage
Store at 20º to 25°C (68º to 77°F). Excursions permitted between 15º to 30°C (59º to 86°F). See USP controlled room temperature. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Instruct patients:
- Administration of two doses of SUTAB (24 tablets) are required for a complete preparation for colonoscopy.
- SUTAB is supplied as two bottles each containing 12 tablets. Twelve (12) tablets are equivalent to one dose.
- Each SUTAB bottle contains a desiccant. Remove and discard the desiccantfrom both bottles of SUTAB the evening prior to the colonoscopy [see Dosage and Administration ( 2.2)] .
- Must consume water with each dose of SUTAB and an additional 32 ounces of water after each dose.
- After breakfast, only clear liquids may be consumed until after the colonoscopy. Examples of clear liquids you can have are coffee or tea (no cream or non-dairy creamer), fruit juices (without pulp), gelatin desserts (no fruit or topping), water, chicken broth, clear soda (such as ginger ale).
- If preparation-related symptoms occur (e.g., nausea, bloating, cramping), pause or slow the rate of drinking the additional water until symptoms diminish.
- Do not take other laxatives while they are taking SUTAB.
- Do not drink milk or eat or drink anything colored red or purple.
- Do not drink alcohol.
- Administer oral medications at least one hour before starting each dose of SUTAB.
- If taking tetracycline or fluoroquinolone antibiotics, iron, digoxin, chlorpromazine, or penicillamine, take these medications at least 2 hours before and not less than 6 hours after administration of each dose of SUTAB.
- Complete all SUTAB tablets and required water at least two hours prior to colonoscopy.
- Contact their healthcare provider if they develop significant vomiting or signs of dehydration after taking SUTAB or if they experience cardiac arrhythmias or seizures [see Warnings and Precautions ( 5.1, 5.2, 5.3)] .
- Seek immediate medical care should signs or symptoms of a hypersensitivity reaction occur [see Warnings and Precautions ( 5.7)]
Manufactured by:
Braintree Laboratories, Inc.
270 Centre Street
Holbrook, MA 02343Please see www.sebelapharma.com for patent information.
© Braintree Laboratories, Inc. -
MEDICATION GUIDE
MEDICATION GUIDE
SUTAB ®(Sootab)
(sodium sulfate, potassium sulfate and potassium chloride)
tablets, for oral use
Read and understand these Medication Guide Instructions at least 2 days beforeyour colonoscopy and again before you start taking SUTAB. What is the most important information I should know about SUTAB?
SUTAB and other bowel preparations can cause serious side effects, including:
Serious loss of body fluid (dehydration) and changes in body salts (electrolytes) in your blood.
These changes can cause:- abnormal heartbeats that can cause death.
- seizures.This can happen even if you have never had a seizure.
- kidney problems.
Your chance of having fluid loss and changes in body salts with SUTAB is higher if you:
- have heart problems.
- have kidney problems.
- take water pills (diuretics) or non-steroidal anti-inflammatory drugs (NSAIDs).
Tell your healthcare provider right away if you have any of these symptoms of a loss of too much body fluid (dehydration) while taking SUTAB:
- vomiting urinating less often than normal
- dizziness headache
See “What are the possible side effects of SUTAB?” for more information about side effects.
What is SUTAB?
SUTAB is a prescription medicine used by adults to clean the colon before a colonoscopy. SUTAB cleans your colon by causing you to have diarrhea. Cleaning your colon helps your healthcare provider see the inside of your colon more clearly during your colonoscopy.
It is not known if SUTAB is safe and effective in children.Do nottake SUTAB if your healthcare provider has told you that you have:
- a blockage in your bowel (obstruction) or a problem with food moving too slowly through your intestines (ileus).
- an opening in the wall of your stomach or intestine (bowel perforation).
- a very dilated intestine (toxic colitis or toxic megacolon).
- problems with food and fluid emptying from your stomach (gastric retention).
- allergies to any ingredient in SUTAB. See the end of this Medication Guide for a complete list of ingredients in SUTAB.
Before taking SUTAB, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems.
- have stomach or bowel problems including ulcerative colitis.
- have problems with swallowing or gastric reflux.
- have a history of seizures.
- are withdrawing from drinking alcohol or from taking benzodiazepines.
- have a low blood salt (sodium) level.
- have kidney problems.
- are pregnant. It is not known if SUTAB will harm your unborn baby. Talk to your healthcare provider if you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if SUTAB passes into your breast milk. You and your healthcare provider should decide if you will take SUTAB while breastfeeding.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements.
SUTAB may affect how other medicines work. Medicines taken by mouth may not be absorbed properly when taken within 1 hour before the start of each dose of SUTAB.
Especially tell your healthcare provider if you take:- medicines for blood pressure or heart problems.
- medicines for kidney problems.
- medicines for seizures.
- water pills (diuretics).
- non-steroidal anti-inflammatory drugs (NSAIDs).
- medicines for depression or other mental health problems.
- laxatives. Do nottake other laxatives while taking SUTAB.
The following medicines should be taken at least 2 hours before starting each dose of SUTAB and not less than 6 hours after taking each dose of SUTAB:
tetracycline iron chlorpromazine
fluoroquinolone antibiotics digoxin penicillamine
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure if you are taking any of the medicines listed above.Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take SUTAB?
See the Instructions for Use for dosing instructions. You must read, understand, and follow these instructions to take SUTAB the right way.- Take SUTAB exactly as your healthcare provider tells you to take it.
- Each bottle contains 12 tablets and a drying agent (desiccant) canister to keep the tablets dry. Remove and throw away (discard) the drying agent (desiccant) canister from both bottles. Use the second bottle for the second dose on the morning of the colonoscopy.
- Each dose of SUTAB is 12 tablets that must be taken while drinking 16 ounces of water. Two doses of SUTAB (24 tablets) are required for a complete colonoscopy preparation.
- It is important for you to drink the additional 32 ounces of water after each dose to prevent fluid loss (dehydration).
- SUTAB is taken using the Split-Dosemethod. See the Instructions for Use for more information.
- All people taking SUTAB should follow these general instructions starting 1 day before your colonoscopy:
o you can eat a low-residue breakfast. Low-residue foods include eggs, white bread, cottage cheese, yogurt, grits, coffee and tea.
o after breakfast only drink clear liquids all day and the next day until 2 hours before your colonoscopy. Stop drinking all fluids at least 2 hours before the colonoscopy.
o after taking the first dose of SUTAB (12 tablets) if you have any bloating or feeling like your stomach is upset, wait to take your second dose of 12 tablets until your stomach feels better. - While taking SUTAB,
do not:
o take any other laxatives.
o take any medicines by mouth (oral) within 1 hour of starting SUTAB.
o eat solid foods, dairy such as milk, or drink alcohol.
o eat or drink anything colored red or purple.
What are the possible side effects of SUTAB?
SUTAB can cause serious side effects, including:- See What is the most important information I should know about SUTAB?
-
Changes in certain blood tests.Your healthcare provider may do blood tests after you take SUTAB to check your blood for changes. Tell your healthcare provider if you have any symptoms of too much fluid loss, including:
o vomiting o nausea o bloating
o dizziness o stomach (abdominal) o headache
o urinate less than usual cramping
o trouble drinking clear liquid - Heart problems. SUTAB may cause abnormal heartbeats.
- Seizures.
- Ulcers of the bowel or bowel problems (ischemic colitis).Tell your healthcare provider right away if you have severe stomach-area (abdominal) pain or rectal bleeding.
-
Serious allergic reactions.Get medical help right away if you have any signs and symptoms of a serious allergic reaction while taking SUTAB, including:
o Swelling of your hands, face o rash
lips, mouth or tongue o severe itching
o breathing problems o raised red patches on your skin
The most common side effects of SUTAB include:
nausea vomiting
stomach-area swelling (abdominal distention) upper stomach (abdominal) pain
These are not all the possible side effects of SUTAB.Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800- FDA-1088.
How should I store SUTAB?
Each SUTAB bottle contains a drying agent (desiccant) canister to help keep the tablets dry (protect from moisture).
Store SUTAB at room temperature between 68°F to 77°F (20°C to 25°C).
Keep SUTAB and all medicines out of the reach of children.
General information about the safe and effective use of SUTAB.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use SUTAB for a condition for which it was not prescribed. Do not give SUTAB to other people, even if they are going to have the same procedure you are. It may harm them.
You can ask your pharmacist or healthcare provider for information that is written for health professionals.
What are the ingredients in SUTAB?
Active ingredients:sodium sulfate, magnesium sulfate, and potassium chloride.
Inactive ingredients: polyethylene glycol 8000, sodium caprylate, and ethylene glycol and vinyl alcohol graft copolymer
Manufactured by:
Braintree Laboratories, Inc.
270 Centre Street
Holbrook, MA 02343
For more information, go to www.braintreelabs.com or call 1-800-874-6756.This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 10/2023
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Carton Label
NDC: 52268-201-01
Please see www.sebelapharma.com for patent information.
SUTAB
(sodium sulfate, magnesium sulfate, and potassium chloride)
Tablets
1.479g/0.225g/0.188gNOTE TO PHARMACIST:Inform patients to REMOVE AND DISCARD the DESICCANTfrom both medication bottles before SUTAB ingestion. This carton contains:
2Bottles of 12 tablets each
116-ounce cup
1Patient booklet
Booklet includes:
1. Instructions for Use
2. Full Prescribing Information
3. Medication GuideBoth 12-tablet bottles are required for a complete preparation.
©2020 Braintree Laboratories Inc. All rights reserved. OCT 2023
SUT20101Rx only
Braintree
A PART OF SEBELA PHARMACEUTICALS
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Bottle Label
NDC: 52268-200-01
Rx onlySUTAB
(sodium sulfate, magnesium sulfate, and potassium chloride)
Tablets
1.479g/0.225g/0.188gREMOVE and DISCARD DESICCANT This bottle contains 12 tablets and a desiccant.
Both 12-tablet bottles are required for a complete preparation.
Store at 25°C (77°F);
excursions permitted
15-30°C (59-86°F)Recommended Dosage:
See full prescribing information©2020 Manufactured by Braintree Laboratories, Inc. Braintree, MA
-
INGREDIENTS AND APPEARANCE
SUTAB
sodium sulfate, magnesium sulfate, and potassium chloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52268-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 17.75 g MAGNESIUM SULFATE ANHYDROUS (UNII: ML30MJ2U7I) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE ANHYDROUS 2.7 g POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CATION 2.25 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) SODIUM CAPRYLATE (UNII: 9XTM81VK2B) ETHYLENE GLYCOL (UNII: FC72KVT52F) POLYVINYL ALCOHOL GRAFT POLYETHYLENE GLYCOL COPOLYMER (3:1; 45000 MW) (UNII: 23ZQ42JZZH) Product Characteristics Color white (white to off-white) Score no score Shape OVAL Size 19mm Flavor Imprint Code S24 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52268-201-01 2 in 1 KIT 11/10/2020 1 12 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213135 11/10/2020 Labeler - Braintree Laboratories, Inc. (107904591) Establishment Name Address ID/FEI Business Operations Braintree Laboratories, Inc. 617357954 manufacture(52268-201) , analysis(52268-201)
Trademark Results [SUTAB]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SUTAB 88656899 not registered Live/Pending |
Sebela BT Holdings, Inc. 2019-10-16 |
 SUTAB 88656848 not registered Live/Pending |
Sebela BT Holdings, Inc. 2019-10-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.