FLUZONE HIGH DOSE NORTHERN HEMISPHERE (influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/croatia/10136/rv/2023 (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen- formaldehyde inactivated injection, suspension
FLUZONE HIGH DOSE NORTHERN HEMISPHERE by
Drug Labeling and Warnings
FLUZONE HIGH DOSE NORTHERN HEMISPHERE by is a Other medication manufactured, distributed, or labeled by Sanofi Pasteur Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Fluzone® High-Dose safely and effectively. See full prescribing information for Fluzone High-Dose.
Fluzone High-Dose (Influenza Vaccine)
injectable suspension, for intramuscular use
2025–2026 Formula
Initial U.S. Approval: 2009INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- For intramuscular use
Administer Fluzone High-Dose as a single 0.5 mL dose. (2)
DOSAGE FORMS AND STRENGTHS
Fluzone High-Dose is an injectable suspension. A single dose is 0.5 mL. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- If Guillain-Barré Syndrome (GBS) has occurred within 6 weeks following previous influenza vaccination, the decision to give Fluzone High-Dose should be based on careful consideration of the potential benefits and risks. (5.1)
ADVERSE REACTIONS
- In adults ≥65 years of age, the most common (>10%) injection-site adverse reaction was pain (>35.6%); the most common solicited systemic adverse reactions were myalgia (21.4%), malaise (18.0%), and headache (16.8%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sanofi Pasteur Inc., Discovery Drive, Swiftwater, PA 18370 at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 or https://vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose and Schedule
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
5.2 Preventing and Managing Allergic Reactions
5.3 Altered Immunocompetence
5.4 Limitations of Vaccine Effectiveness
5.5 Syncope
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Immunogenicity of Fluzone High-Dose in Adults 65 Years of Age and Older
14.2 Efficacy of Fluzone High-Dose in Adults 65 Years of Age and Older
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For intramuscular use
2.1 Dose and Schedule
Administer Fluzone High-Dose as a single 0.5 mL dose from one prefilled syringe.
2.2 Administration
Fluzone High-Dose is a colorless opalescent liquid. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exist, the vaccine should not be administered.
Before administering a dose of vaccine, shake the prefilled syringe.
Administer Fluzone High-Dose intramuscularly.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer Fluzone High-Dose to anyone with a history of severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine [see Description (11)], including egg protein, or to a previous dose of any influenza vaccine.
-
5 WARNINGS AND PRECAUTIONS
5.1 Guillain-Barré Syndrome
If Guillain-Barré Syndrome (GBS) has occurred within 6 weeks following previous influenza vaccination, the decision to give Fluzone High-Dose should be based on careful consideration of the potential benefits and risks. The 1976 swine influenza vaccine was associated with an elevated risk of GBS. Evidence for a causal relation of GBS with other influenza vaccines is inconclusive; if an excess risk exists, it is probably slightly more than 1 additional case per 1 million persons vaccinated.(See reference 1.)
5.2 Preventing and Managing Allergic Reactions
Appropriate medical treatment must be immediately available to manage potential anaphylactic reactions following administration of Fluzone High-Dose.
5.3 Altered Immunocompetence
If Fluzone High-Dose is administered to immunocompromised persons, including those receiving immunosuppressive therapy, the expected immune response may not be obtained.
-
6 ADVERSE REACTIONS
In adults ≥65 years of age, the most common (>10%) injection-site adverse reaction was pain (>35.6%); the most common solicited systemic adverse reactions were myalgia (21.4%), malaise (18.0%), and headache (16.8%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse event rates observed in the clinical trial(s) of a vaccine cannot be directly compared to rates in the clinical trial(s) of another vaccine and may not reflect the rates observed in practice. Two clinical studies have evaluated the safety of Fluzone High-Dose.
Study 1 (NCT00391053) was a multi-center, double-blind trial conducted in the US. In this study, adults 65 years of age and older were randomized to receive either Fluzone High-Dose or Fluzone (2006–2007 formulation). The study compared the safety and immunogenicity of Fluzone High-Dose to those of Fluzone. The safety analysis set included 2573 Fluzone High-Dose recipients and 1260 Fluzone recipients.
Table 1 summarizes solicited injection-site reactions and systemic adverse reactions reported within 7 days post-vaccination via diary cards. Onset was usually within the first 3 days after vaccination and a majority of the reactions resolved within 3 days. Solicited injection-site reactions and systemic adverse reactions were more frequent after vaccination with Fluzone High-Dose compared to Fluzone.
Table 1: Frequency of Solicited Injection-Site Reactions and Systemic Adverse Reactions Within 7 Days After Vaccination with Fluzone High-Dose or Fluzone, Adults 65 Years of Age and Older (Study 1*) Fluzone High-Dose (N†=2569–2572)
PercentageFluzone (N†=1258–1260)
PercentageAny Moderate‡ Severe§ Any Moderate‡ Severe§ - * NCT00391053
- † N is the number of vaccinated participants with available data for the reactions listed
- ‡ Moderate - Injection-site pain: sufficiently discomforting to interfere with normal behavior or activities; Injection- site erythema and Injection-site swelling: ≥2.5 cm to <5 cm; Fever: >100.4°F to ≤102.2°F; Myalgia, Malaise, and Headache: interferes with daily activities
- § Severe - Injection-site pain: incapacitating, unable to perform usual activities; Injection-site erythema and Injection- site swelling: ≥5 cm; Fever: >102.2°F; Myalgia, Malaise, and Headache: prevents daily activities
- ¶ Fever - The percentage of temperature measurements that were taken by oral route or not recorded were 97.9% and 2.1%, respectively, for Fluzone High-Dose; and 98.6% and 1.4%, respectively, for Fluzone
Injection-Site Pain 35.6 3.7 0.3 24.3 1.7 0.2 Injection-Site Erythema 14.9 1.9 1.8 10.8 0.8 0.6 Injection-Site Swelling 8.9 1.6 1.5 5.8 1.3 0.6 Myalgia 21.4 4.2 1.6 18.3 3.2 0.2 Malaise 18.0 4.7 1.6 14.0 3.7 0.6 Headache 16.8 3.1 1.1 14.4 2.5 0.3 Fever¶ (≥99.5°F) 3.6 1.1 0.0 2.3 0.2 0.1 Within 6 months post-vaccination, 156 (6.1%) Fluzone High-Dose recipients and 93 (7.4%) Fluzone recipients experienced a serious adverse event (SAE). No deaths were reported within 28 days post-vaccination. A total of 23 deaths were reported during Days 29 – 180 post-vaccination:
16 (0.6%) among Fluzone High-Dose recipients and 7 (0.6%) among Fluzone recipients. The majority of these participants had a medical history of cardiac, hepatic, neoplastic, renal, and/or respiratory diseases. These data do not provide evidence for a causal relationship between deaths and vaccination with Fluzone High-Dose.
Study 2 (NCT01427309) was a multi-center, double-blind post- licensure efficacy trial conducted in the US and Canada over two influenza seasons. In this study, adults 65 years of age and older were randomized to receive either Fluzone High-Dose or Fluzone (2011–2012 and 2012–2013 formulations). The study compared the efficacy and safety of Fluzone High-Dose to those of Fluzone. The safety analysis set included 15,992 Fluzone High-Dose recipients and 15,991 Fluzone recipients.
Within the study surveillance period (approximately 6 to 8 months post-vaccination), 1323 (8.3%) Fluzone High-Dose recipients and 1442 (9.0%) Fluzone recipients experienced an SAE. Within 30 days post-vaccination, 204 (1.3%) Fluzone High-Dose recipients and 200 (1.3%) Fluzone recipients experienced an SAE. The majority of these participants had one or more chronic comorbid illnesses. A total of 167 deaths were reported within 6 to 8 months post-vaccination: 83 (0.5%) among Fluzone High-Dose recipients and 84 (0.5%) among Fluzone recipients. A total of 6 deaths were reported within 30 days post-vaccination: 6 (0.04%) among Fluzone High-Dose recipients and 0 (0%) among Fluzone recipients. These data do not provide evidence for a causal relationship between deaths and vaccination with Fluzone High-Dose.
6.2 Postmarketing Experience
The following events have been spontaneously reported during the postmarketing use of Fluzone, Fluzone High-Dose, Fluzone Quadrivalent, or Fluzone High-Dose Quadrivalent. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. Adverse events were included based on one or more of the following factors: severity, frequency of reporting, or strength of evidence for a causal relationship to the vaccine.
- Blood and Lymphatic System Disorders: Thrombocytopenia, lymphadenopathy
- Immune System Disorders: Anaphylaxis, other allergic/hypersensitivity reactions (including urticaria, angioedema)
- Eye Disorders: Ocular hyperemia
- Nervous System Disorders: Guillain-Barré syndrome (GBS), convulsions, febrile convulsions, myelitis (including encephalomyelitis and transverse myelitis), facial palsy (Bell's palsy), optic neuritis/neuropathy, brachial neuritis, syncope (shortly after vaccination), dizziness, paresthesia
- Vascular Disorders: Vasculitis, vasodilatation/flushing
- Respiratory, Thoracic and Mediastinal Disorders: Dyspnea, oropharyngeal pain, rhinorrhea, cough, wheezing, throat tightness
- Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome
- General Disorders and Administration Site Conditions: Pruritus, asthenia/fatigue, pain in extremities, chest pain, chills
- Gastrointestinal Disorders: Vomiting, nausea, diarrhea
- Musculoskeletal and Connective Tissue Disorders: Arthralgia
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Fluzone High-Dose is not approved for use in persons <65 years of age.
There are limited human data and no animal data available to establish whether there is a vaccine-associated risk with use of Fluzone High-Dose in pregnancy.
8.2 Lactation
Fluzone High-Dose is not approved for use in persons <65 years of age. No human or animal data are available to assess the effects of Fluzone High-Dose on the breastfed infant or on milk production/excretion.
8.4 Pediatric Use
Safety and effectiveness of Fluzone High-Dose in persons <65 years of age have not been established.
8.5 Geriatric Use
Safety, immunogenicity, and efficacy of Fluzone High-Dose have been evaluated in adults 65 years of age and older. [See Adverse Reactions (6.1) and Clinical Studies (14)]
-
11 DESCRIPTION
Fluzone High-Dose (Influenza Vaccine) for intramuscular use is an inactivated influenza vaccine, prepared from influenza viruses propagated in embryonated chicken eggs. The virus- containing allantoic fluid is harvested and inactivated with formaldehyde. Influenza virus is concentrated and purified in a linear sucrose density gradient solution using a continuous flow centrifuge. The virus is then chemically disrupted using a non-ionic surfactant, octylphenol ethoxylate (Triton® X-100), producing a "split virus". The split virus containing hemagglutinin (HA) antigen is further purified and then suspended in sodium phosphate-buffered isotonic sodium chloride solution. The Fluzone High-Dose process uses an additional concentration factor after the ultrafiltration step to obtain a higher HA antigen concentration. The purified split virus from the three strains included in the vaccine are produced separately and then combined to make the trivalent formulation.
Fluzone High-Dose is an injectable suspension and is a colorless opalescent liquid.
Neither antibiotics nor preservative are used in the manufacture of Fluzone High-Dose.
The Fluzone High-Dose prefilled syringe presentation is not made with natural rubber latex.
Fluzone High-Dose is standardized according to United States Public Health Service requirements and is formulated to contain HA of each of the following three influenza strains recommended for the 2025–2026 influenza season: A/Victoria/4897/2022 IVR-238 (H1N1), A/Croatia/10136RV/2023 X-425A (H3N2), and B/Michigan/01/2021 (a B/Austria/1359417/2021-like virus, B Victoria lineage). The amounts of HA and other ingredients per dose of vaccine are listed in Table 2.
Table 2: Fluzone High-Dose Ingredients Ingredient Quantity
(per dose)Fluzone High-Dose
0.5 mL Dose- * per United States Public Health Service (USPHS) requirement
- † Quantity Sufficient
Active Substance: Split influenza virus, inactivated strains*: 180 mcg HA total A (H1N1) 60 mcg HA A (H3N2) 60 mcg HA B 60 mcg HA Other: Sodium phosphate-buffered isotonic sodium chloride solution QS† to appropriate volume Formaldehyde ≤100 mcg Octylphenol ethoxylate ≤250 mcg Gelatin None Preservative None -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Specific levels of hemagglutination inhibition (HI) antibody titer post-vaccination with inactivated influenza virus vaccines have not been correlated with protection from influenza virus infection. In some human studies, antibody titers ≥1:40 have been associated with protection from influenza illness in up to 50% of participants. (See references 2 and 3.)
Antibodies against one influenza virus type or subtype confer limited or no protection against another. Furthermore, antibodies to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype. Frequent development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the usual change of one or more new strains in each year's influenza vaccine.
- 13 NON-CLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Immunogenicity of Fluzone High-Dose in Adults 65 Years of Age and Older
Study 1 was a multi-center, double-blind pre-licensure trial conducted in the US in which adults 65 years of age and older were randomized to receive either Fluzone High-Dose or Fluzone (2006–2007 formulation). The study compared the safety and immunogenicity of Fluzone High-Dose to those of Fluzone. For immunogenicity analyses, 2576 participants were randomized to Fluzone High-Dose and 1275 participants were randomized to Fluzone. Females accounted for 51.3% of participants in the Fluzone High-Dose group and 54.7% of participants in the Fluzone group. In both groups, the mean age was 72.9 years (ranged from 65 through 97 years in the Fluzone High-Dose group and 65 through 94 years in the Fluzone group); 35% of participants in the Fluzone High-Dose group and 36% of participants in the Fluzone group were 75 years of age or older. Most participants in the Fluzone High-Dose and Fluzone groups, respectively, were White (91.7% and 92.9%), followed by Hispanic (4.8% and 3.7%), and Black (2.7% and 2.7%).
The primary endpoints of the study were HI GMTs and seroconversion rates 28 days after vaccination. Pre-specified statistical superiority criteria required that the lower limit (LL) of the 2- sided 95% CI of the GMT ratio (Fluzone High-Dose/Fluzone) be greater than 1.50 for at least two of the strains, and if one strain failed, non-inferiority of that strain must be demonstrated (LL>0.67), and that the lower limit of the 2-sided 95% CI of the seroconversion rate difference (Fluzone High-Dose minus Fluzone) be greater than 10% for at least two of the strains, and if one strain failed, non-inferiority of that strain must be demonstrated (LL>-10%). As shown in Table 3, statistically superior HI GMTs and seroconversion rates after vaccination with Fluzone High-Dose compared to Fluzone were demonstrated for influenza A subtypes, A (H1N1) and A (H3N2), but not for influenza type B. For strain B, non-inferiority of Fluzone High-Dose compared to Fluzone was demonstrated for both the HI GMTs and seroconversion rates.
Table 3: Post-Vaccination HI Antibody GMTs and Seroconversion Rates and Analyses of Superiority of Fluzone High-Dose Relative to Fluzone, Adults 65 Years of Age and Older (Study 1*) Influenza Strain GMT GMT Ratio Seroconversion %† Difference Met Both Pre-defined Superiority Criteria‡ Fluzone High-Dose
N§=2542–2544Fluzone
N§=1252Fluzone High-Dose over Fluzone
(95% CI)Fluzone High-Dose
N§=2529–2531Fluzone
N§=1248–1249Fluzone High-Dose minus Fluzone
(95% CI)- * NCT00391053
- † Seroconversion: Paired samples with pre-vaccination HI titer <1:10 and post-vaccination (day 28) titer ≥1:40 or a minimum 4-fold increase for participants with pre-vaccination titer ≥1:10
- ‡ Predefined superiority criterion for seroconversion: the lower limit of the two-sided 95% CI of the difference of the seroconversion rates (Fluzone High-Dose minus Fluzone) is >10%. Predefined superiority criterion for the GMT ratio: the lower limit of the 95% CI of the GMT ratio (Fluzone High-Dose divided by Fluzone) is >1.5
- § N is the number of vaccinated participants with available data for the immunologic endpoint listed
A (H1N1) 115.8 67.3 1.7 (1.6; 1.8) 48.6 23.1 25.4 (22.4; 28.5) Yes A (H3N2) 608.9 332.5 1.8 (1.7; 2.0) 69.1 50.7 18.4 (15.1; 21.7) Yes B 69.1 52.3 1.3 (1.2; 1.4) 41.8 29.9 11.8 (8.6; 15.0) No 14.2 Efficacy of Fluzone High-Dose in Adults 65 Years of Age and Older
Study 2 was a multi-center, double-blind post-licensure efficacy trial conducted in the US and Canada in which adults 65 years of age and older were randomized (1:1) to receive either Fluzone High-Dose or Fluzone. The study was conducted over two influenza seasons (2011–2012 and 2012–2013); 53% of participants enrolled in the first year of the study were re- enrolled and re-randomized in the second year. The per-protocol analysis set for efficacy assessments included 15,892 Fluzone High-Dose recipients and 15,911 Fluzone recipients. The majority (67%) of participants in the per-protocol analysis set for efficacy had one or more high- risk chronic comorbid conditions.
In the per-protocol analysis set, females accounted for 57.2% of participants in the Fluzone High- Dose group and 56.1% of participants in the Fluzone group. In both groups, the median age was 72.2 years (range 65 through 100 years). Overall, most participants in the study were White (95%); approximately 4% of study participants were Black, and approximately 6% reported Hispanic ethnicity.
The primary endpoint of the study was the occurrence of laboratory-confirmed influenza (as determined by culture or polymerase chain reaction) caused by any influenza viral type/subtype in association with influenza-like illness (ILI), defined as the occurrence of at least one of the following respiratory symptoms: sore throat, cough, sputum production, wheezing, or difficulty breathing; concurrent with at least one of the following systemic signs or symptoms: temperature >99.0°F, chills, tiredness, headaches or myalgia. Participants were monitored for the occurrence of a respiratory illness by both active and passive surveillance, starting 2 weeks post-vaccination for approximately 7 months. After an episode of respiratory illness, nasopharyngeal swab samples were collected for analysis; attack rates and vaccine efficacy were calculated (see Table 4).
Table 4: Relative Efficacy Against Laboratory-Confirmed Influenza* Regardless of Similarity to the Vaccine Components, Associated with Influenza-Like Illness†, Adults 65 Years of Age and Older (Study 2‡) Fluzone High-Dose
N§=15,892
n¶ (%)Fluzone
N§=15,911
n¶ (%)Relative Efficacy
% (95% CI)- * Laboratory-confirmed: culture- or polymerase-chain-reaction-confirmed
- † Occurrence of at least one of the following respiratory symptoms: sore throat, cough, sputum production, wheezing, or difficulty breathing; concurrent with at least one of the following systemic signs or symptoms: temperature >99.0°F, chills, tiredness, headaches or myalgia
- ‡ NCT01427309
- § N is the number of vaccinated participants in the per-protocol analysis set for efficacy assessments
- ¶ n is the number of participants with protocol-defined influenza-like illness with laboratory confirmation
- # Primary endpoint
- Þ The pre-specified statistical superiority criterion for the primary endpoint (lower limit of the 2-sided 95% CI of the vaccine efficacy of Fluzone High-Dose relative to Fluzone > 9.1%) was met.
- ß In the first year of the study the influenza B component of the vaccine and the majority of influenza B cases were of the Victoria lineage; in the second year the influenza B component of the vaccine and the majority of influenza B cases were of the Yamagata lineage
Any type/subtype# 227 (1.43) 300 (1.89) 24.2 (9.7; 36.5) Þ Influenza A 190 (1.20) 249 (1.56) 23.6 (7.4; 37.1) A (H1N1) 8 (0.05) 9 (0.06) 11.0 (-159.9; 70.1) A (H3N2) 171 (1.08) 222 (1.40) 22.9 (5.4; 37.2) Influenza Bß 37 (0.23) 51 (0.32) 27.4 (-13.1; 53.8) A secondary endpoint of the study was the occurrence of culture-confirmed influenza caused by viral types/subtypes antigenically similar to those contained in the respective annual vaccine formulations in association with a modified CDC-defined ILI, defined as the occurrence of a temperature >99.0°F (>37.2°C) with cough or sore throat. The efficacy of Fluzone High-Dose relative to Fluzone for this endpoint was 51.1% (95% CI: 16.8; 72.0).
-
15 REFERENCES
- 1 Lasky T, Terracciano GJ, Magder L, et al. The Guillain-Barré syndrome and the 1992–1993 and 1993–1994 influenza vaccines. N Engl J Med 1998;339:1797–802.
- 2 Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004;103:133–138.
- 3 Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination- inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg Camb 1972;70:767–777.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Single-dose, prefilled syringe, without needle, 0.5 mL (NDC: 49281-125-88) (not made with natural rubber latex). Supplied as a package (NDC: 49281-125-65) of 10 prefilled syringes presented as:
- 2 rows of 5 single-dose prefilled syringes separated by a cardboard partition. Use one syringe per dose, or
- 5 sleeves, with each sleeve containing 2 single-dose prefilled syringes. Use one syringe per dose.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
- Inform the patient or caregiver that Fluzone High-Dose contains killed viruses and cannot cause influenza.
- Fluzone High-Dose stimulates the immune system to produce antibodies that help protect against influenza.
- Instruct that annual influenza vaccination is recommended.
- Instruct vaccine recipients and caregivers to report adverse reactions to their healthcare provider and/or to Vaccine Adverse Event Reporting System (VAERS).
- Give the Vaccine Information Statements to recipients or caregivers, which are required by the National Childhood Vaccine Injury Act of 1986 prior to each immunization. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
Patient Information Sheet
Fluzone® High-Dose
Influenza VaccinePlease read this information sheet before getting Fluzone High-Dose vaccine. This summary is not intended to take the place of talking with your healthcare provider. If you have questions or would like more information, please talk with your healthcare provider.
What is Fluzone High-Dose vaccine?
Fluzone High-Dose is a vaccine that helps protect against influenza illness (flu).
Fluzone High-Dose vaccine is for people 65 years of age and older.
Vaccination with Fluzone High-Dose vaccine may not protect all people who receive the vaccine.
Who should not get Fluzone High-Dose vaccine?
You should not get Fluzone High-Dose vaccine if you:
- ever had a severe allergic reaction to eggs or egg products.
- ever had a severe allergic reaction after getting any influenza vaccine.
Tell your healthcare provider if you have or have had:
- Guillain-Barré syndrome (severe muscle weakness) after getting an influenza vaccine.
- problems with your immune system as the immune response may be diminished.
How is Fluzone High-Dose vaccine given?
Fluzone High-Dose vaccine is given as an injection into the muscle.
What are the possible side effects of Fluzone High-Dose vaccine?
The most common side effects of Fluzone High-Dose vaccine are:
- pain, redness, and swelling where you got the injection
- muscle ache
- tiredness
- headache
These are not all of the possible side effects of Fluzone High-Dose vaccine. Ask your healthcare provider about other side effects.
Call your healthcare provider for advice about any side effects that concern you. You may report side effects to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 or https://vaers.hhs.gov.
Why should I get Fluzone High-Dose vaccine instead of Fluzone vaccine?
An efficacy study in adults 65 years of age and older has demonstrated that Fluzone High-Dose vaccine offers better protection against influenza than Fluzone vaccine.
What are the ingredients in Fluzone High-Dose vaccine?
Fluzone High-Dose vaccine contains 3 killed influenza virus strains. There is no live influenza virus in Fluzone High-Dose. Fluzone High-Dose cannot cause influenza.
Other ingredients include formaldehyde and octylphenol ethoxylate.
Manufactured by: Sanofi Pasteur Inc.
Swiftwater, PA 18370 USA -
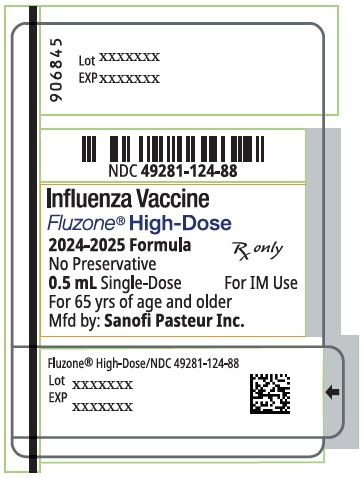
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Label
934378
Lot XXXXXXX
EXP XXXX/XXNDC: 49281-125-88
Influenza Vaccine
Fluzone® High-Dose
2025-2026 Formula
No Preservative
0.5 mL Single-Dose
For IM UseFor 65 yrs of age and older
Mfd by: Sanofi Pasteur Inc.
Rx only
-
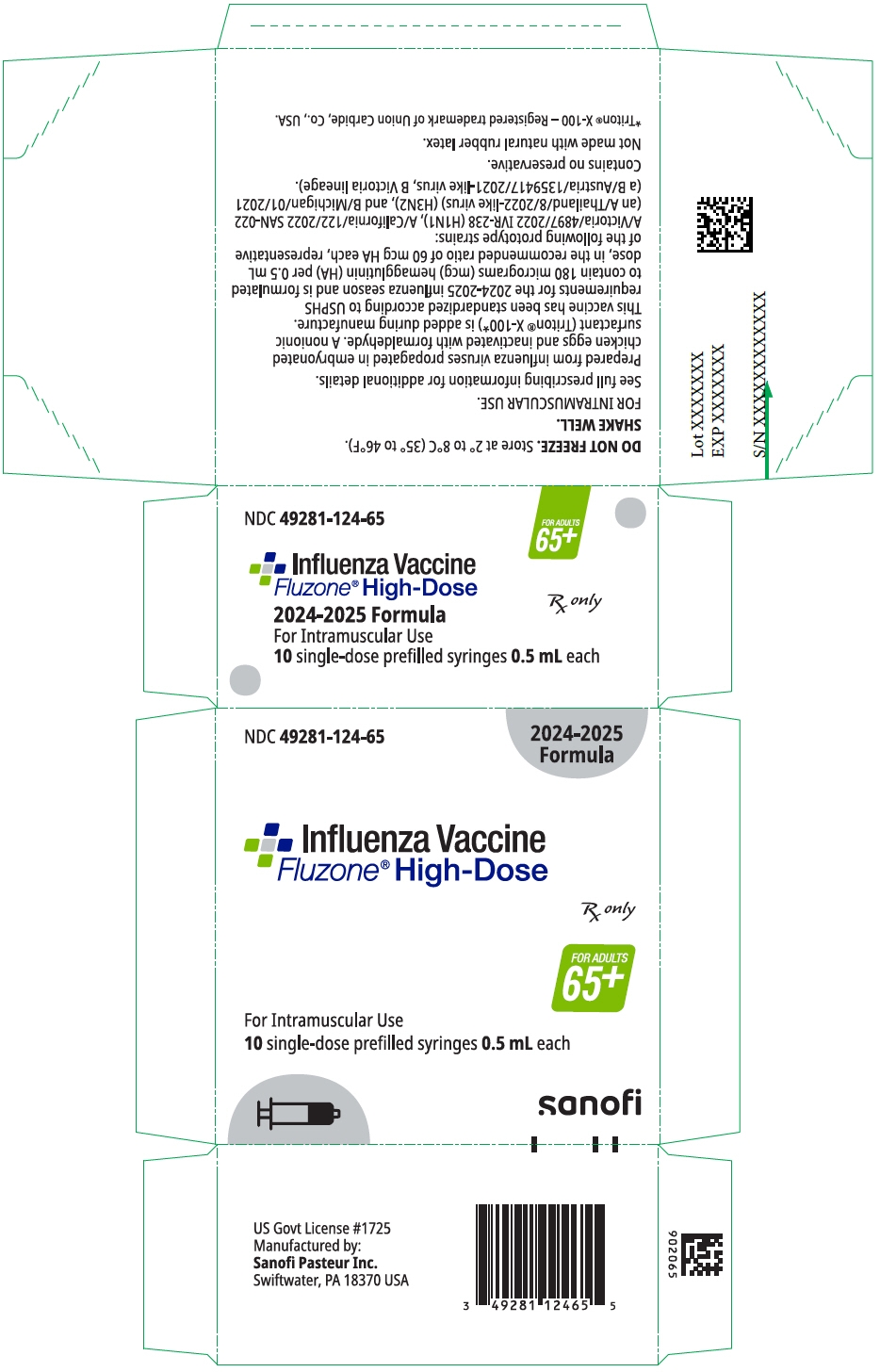
PRINCIPAL DISPLAY PANEL - 0.5 mL Syringe Package
NDC: 49281-125-65
2025-2026
FormulaInfluenza Vaccine
Fluzone® High-DoseRx only
FOR ADULTS
65+For Intramuscular Use
10 single-dose prefilled syringes 0.5 mL eachsanofi
-
INGREDIENTS AND APPEARANCE
FLUZONE HIGH DOSE NORTHERN HEMISPHERE
influenza a virus a/victoria/4897/2022 ivr-238 (h1n1) antigen (formaldehyde inactivated), influenza a virus a/croatia/10136/rv/2023 (h3n2) antigen (formaldehyde inactivated), and influenza b virus b/michigan/01/2021 antigen (formaldehyde inactivated) injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC: 49281-125 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: AU5C98U4BB) (INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:C46XJT9FQ9) INFLUENZA A VIRUS A/VICTORIA/4897/2022 IVR-238 (H1N1) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 60 ug in 0.5 mL INFLUENZA A VIRUS A/CROATIA/10136RV/2023 X-425A (H3N2) ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: HQW9FZS4YK) (INFLUENZA A VIRUS A/CROATIA/10136RV/2023 X-425A (H3N2) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:SD82XNG5M6) INFLUENZA A VIRUS A/CROATIA/10136RV/2023 X-425A (H3N2) HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 60 ug in 0.5 mL INFLUENZA B VIRUS B/MICHIGAN/01/2021 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: FF9YP4D23C) (INFLUENZA B VIRUS B/MICHIGAN/01/2021 HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:CQV855H5FG) INFLUENZA B VIRUS B/MICHIGAN/01/2021 HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 60 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) WATER (UNII: 059QF0KO0R) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) FORMALDEHYDE (UNII: 1HG84L3525) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-125-65 5 in 1 PACKAGE 1 NDC: 49281-125-88 0.5 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103914 07/01/2025 06/30/2026 Labeler - Sanofi Pasteur Inc. (086723285) Registrant - Sanofi Pasteur Inc. (086723285) Establishment Name Address ID/FEI Business Operations Sanofi Pasteur Inc. 086723285 MANUFACTURE(49281-125) , PACK(49281-125) , LABEL(49281-125)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.