Sweet Balm by isamogu Inc. / Isamogu Inc. Sweet Balm 82690-302
Sweet Balm by
Drug Labeling and Warnings
Sweet Balm by is a Otc medication manufactured, distributed, or labeled by isamogu Inc., Isamogu Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
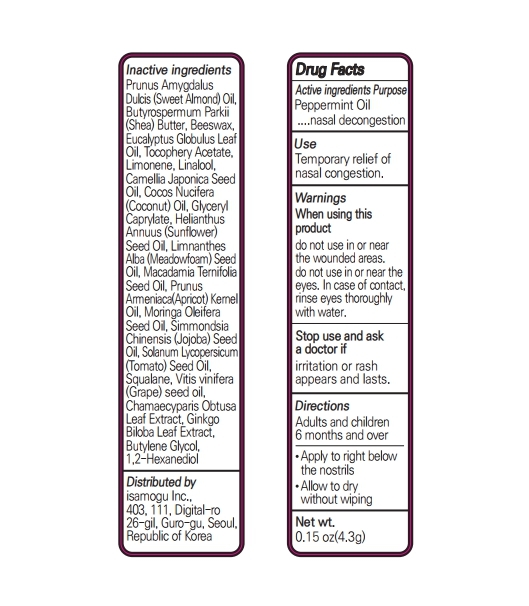
SWEET BALM- peppermint oil stick
isamogu Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Sweet Balm 82690-302
Directions
Adults and children 6 months and over.
Apply to right below the nostrils.
Allow to dry without wiping.
Inactive Ingredients
Prunus AmygdalusDulcis (Sweet Almond) Oil, Butyrospermum Parkii (Shea) Butter, Beeswax, Eucalyptus Globulus Leaf Oil, Tocophery Acetate, Limonene, Linalool, Camellia Japonica Seed Oil, Cocos Nucifera (Coconut) Oil, Glyceryl Caprylate, Helianthus Annuus (Sunflower) Seed Oil, Limnanthes Alba (Meadowfoam) Seed Oil, Macadamia Ternifolia Seed Oil, Prunus Armeniaca(Apricot) Kernel Oil, Moringa Oleifera Seed Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Solanum Lycopersicum (Tomato) Seed Oil, Squalane, Vitis vinifera (Grape) seed oil, Chamaecyparis Obtusa Leaf Extract, Ginkgo Biloba Leaf Extract, Butylene Glycol, 1,2-Hexanediol
| SWEET BALM
peppermint oil stick |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - isamogu Inc. (695695834) |
| Registrant - isamogu Inc. (695695834) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Isamogu Inc. | 695695834 | manufacture(82690-302) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.