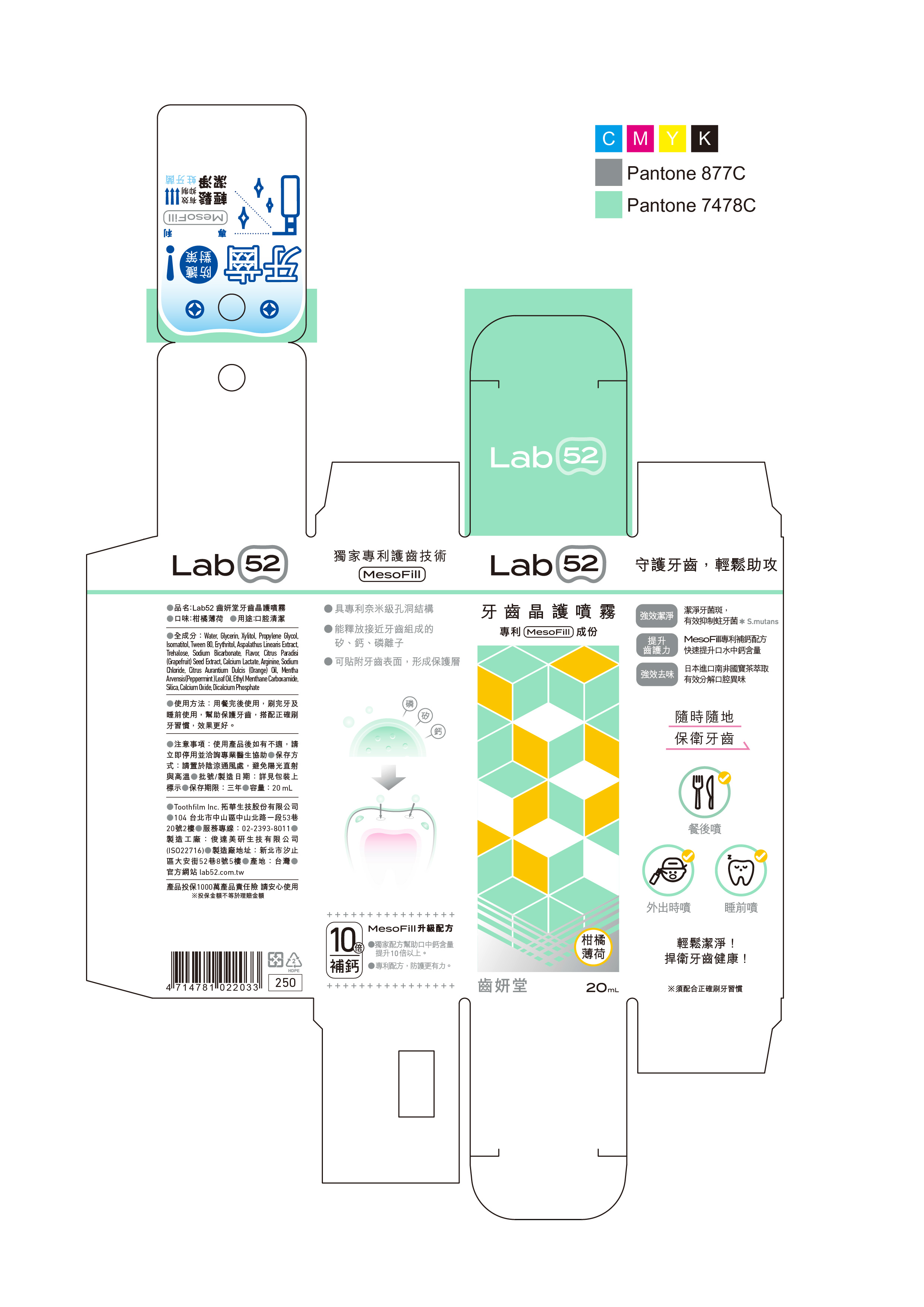
Lab52 Anti-Cavity Oral Care by TOOTHFILM INC
Lab52 Anti-Cavity Oral Care by
Drug Labeling and Warnings
Lab52 Anti-Cavity Oral Care by is a Otc medication manufactured, distributed, or labeled by TOOTHFILM INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LAB52 ANTI-CAVITY ORAL CARE- xylitol, calcium lactate aerosol
TOOTHFILM INC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Water, Glycerin, Propylene Glycol, Erythritol, Sodium Levulinate, Sodium Anisate, Polysorbate 80, Orange Flavor, Citrus Aurantium Dulcis (Orange) Oil, Aspalathus Linearis Extract, Trehalose, Sodium Bicarbonate, Ethyl Menthane Carboxamide, Mentha Arvensis(Peppermint )Leaf Oil, Silica, Calcium Oxide, Dicalcium PhosphateWater, Glycerin, Propylene Glycol, Erythritol, Sodium Levulinate, Sodium Anisate, Polysorbate 80, Orange Flavor, Citrus Aurantium Dulcis (Orange) Oil, Aspalathus Linearis Extract, Trehalose, Sodium Bicarbonate, Ethyl Menthane Carboxamide, Mentha Arvensis(Peppermint )Leaf Oil, Silica, Calcium Oxide, Dicalcium Phosphate
If you feel uncomfortable after using the product, please stop using it immediately and consult a professional doctor for assistance
Use after meals, after brushing your teeth and before going to bed to help protect your teeth, with correct brushing habits, the effect is better
Please keep out of reach of children under 6 years old.
Children under six years of age must be supervised by an adult
| LAB52 ANTI-CAVITY ORAL CARE
xylitol, calcium lactate aerosol |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - TOOTHFILM INC (656036549) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TOOTHFILM INC | 656036549 | manufacture(82711-001) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.