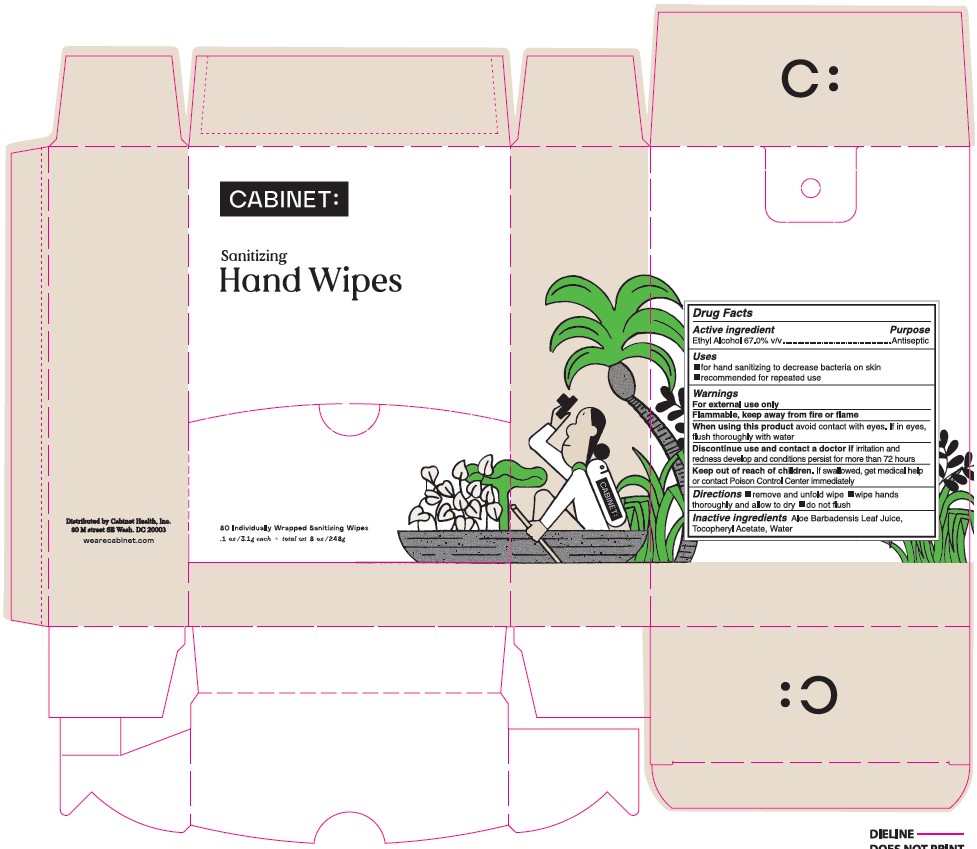
Sanitizer Hand Wipes by Hand Sanitizer / Diamond Wipes International, Inc. Sanitizing Hand wipes
Sanitizer Hand Wipes by
Drug Labeling and Warnings
Sanitizer Hand Wipes by is a Otc medication manufactured, distributed, or labeled by Hand Sanitizer, Diamond Wipes International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SANITIZER HAND WIPES- alcohol cloth
Hand Sanitizer
----------
Sanitizing Hand wipes
Warnings
For external use only
Flammable, Keep away from fire or flame
When using this product avoid contact with eyes, if in eyes, flush thoroughly with water
Discontinue use and contact a doctor if irritation and redness develop and conditions persists for more than 72 hours
| SANITIZER HAND WIPES
alcohol cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hand Sanitizer (117102391) |
| Registrant - Diamond Wipes International, Inc. (161104729) |
Revised: 8/2024
Document Id: 20d621b6-b7b3-822e-e063-6294a90aaeeb
Set id: de31a82a-b7be-f24e-e053-2995a90a8514
Version: 3
Effective Time: 20240829
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.