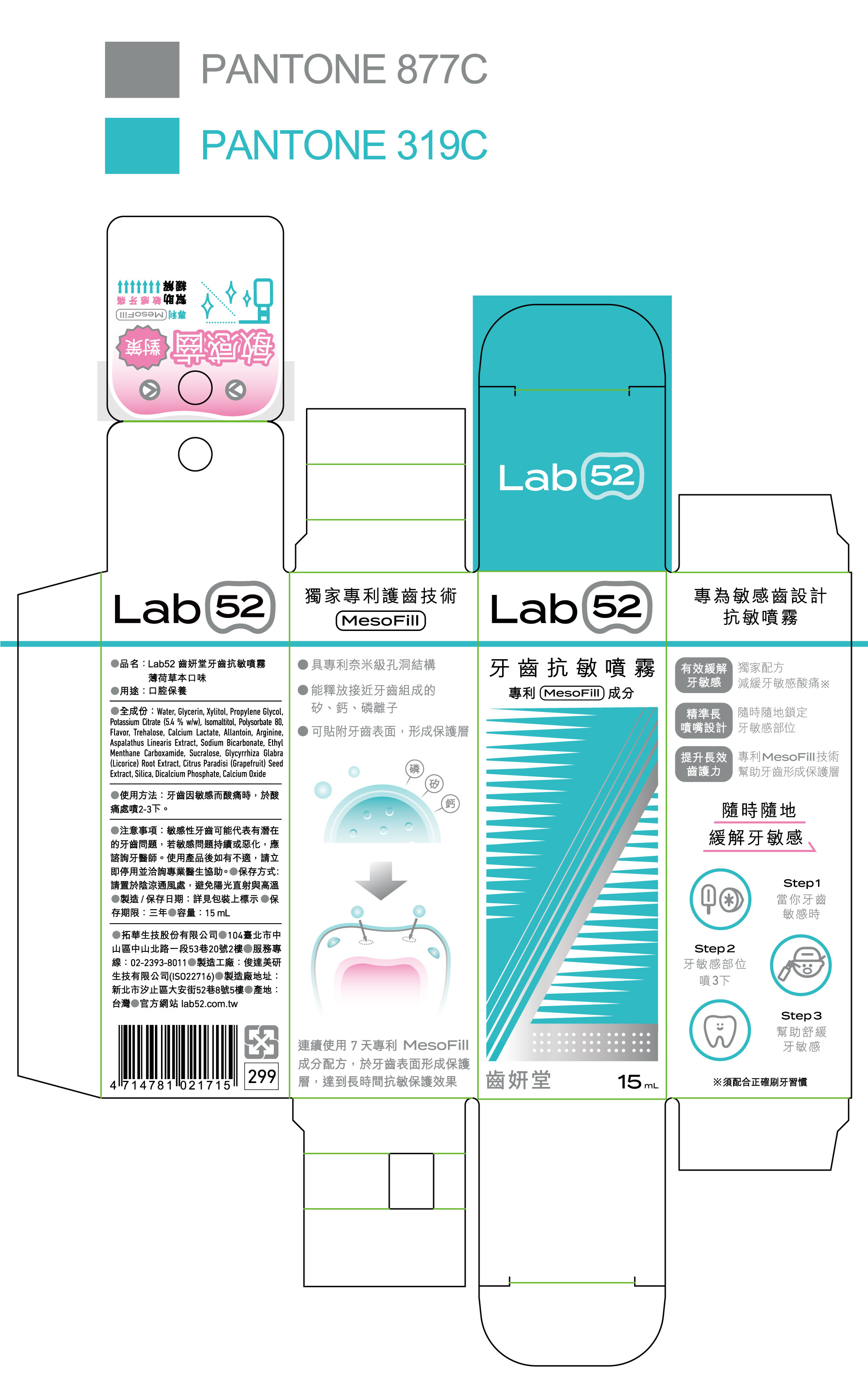
Lab52 Anti-Sensitivity Oral Care by TOOTHFILM INC Marketing End 002
Lab52 Anti-Sensitivity Oral Care by
Drug Labeling and Warnings
Lab52 Anti-Sensitivity Oral Care by is a Otc medication manufactured, distributed, or labeled by TOOTHFILM INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LAB52 ANTI-SENSITIVITY ORAL CARE- xylitol, calcium lactate, potassium citrate aerosol
TOOTHFILM INC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Marketing End 002
Water, Glycerin, Propylene Glycol, Isomaltitol, Polysorbate 80, Lemon Flavor, Trehalose, Allantoin, Arginine, Aspalathus Linearis Extract, Sodium Bicarbonate, Ethyl Menthane Carboxamide, Sucralose, Glycyrrhiza Glabra (Licorice) Root Extract, Silica, Dicalcium Phosphate, Calcium Oxide
If you feel uncomfortable after using the product, please stop using it immediately and consult a professional doctor for assistance
Please keep out of reach of children under 6 years old.
Children under six years of age must be supervised by an adult
| LAB52 ANTI-SENSITIVITY ORAL CARE
xylitol, calcium lactate, potassium citrate aerosol |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - TOOTHFILM INC (656036549) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TOOTHFILM INC | 656036549 | manufacture(82711-002) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.