ALBUTEIN (albumin- human injection, solution
ALBUTEIN by
Drug Labeling and Warnings
ALBUTEIN by is a Other medication manufactured, distributed, or labeled by GRIFOLS USA, LLC, Grifols Biologicals LLC, Grifols Therapeutics LLC, INSTITUTO GRIFOLS SA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ALBUTEIN 25% safely and effectively. See full prescribing information for ALBUTEIN 25%.
ALBUTEIN 25% (albumin [human] U.S.P.)
25% solution
Initial U.S. Approval: 1978INDICATIONS AND USAGE
ALBUTEIN 25% is an albumin solution indicated for:
- Hypovolemia. (1.1)
- Cardiopulmonary bypass procedures. (1.2)
- Acute nephrosis. (1.3)
- Hypoalbuminemia. (1.4)
- Ovarian hyperstimulation syndrome. (1.5)
- Neonatal hyperbilirubinemia. (1.6)
- Adult respiratory distress syndrome (ARDS). (1.7)
- Prevention of central volume depletion after paracentesis due to cirrhotic ascites. (1.8)
DOSAGE AND ADMINISTRATION
For Intravenous Use Only
Dosage and infusion rate should be adjusted to the patient's individual requirements.
Indication Dose Hypovolemia Adults:
Initial dose of 25 g (including renal dialysis).
For acute liver failure: initial dose of 12 to 25 g. (2.1)Cardiopulmonary bypass procedures Adults: Initial dose of 25 g. (2.1) Acute nephrosis Adults: 25 g together with diuretic once a day for 7 - 10 days. (2.1) Hypoalbuminemia Adults: 50 to 75 g
For pre- and post-operative hypoproteinemia: 50 to 75 g.
For burn therapy after the first 24 h: initial dose of 25 g and dose adjustment to maintain plasma protein concentration of 2.5 g per 100mL.
Third space protein loss due to infection: initial dose of 50 to 100 g. (2.1)Ovarian hyperstimulation syndrome Adults: 50 g to 100 g over 4 hours and repeated at 4-12 hour intervals as necessary. (2.1) Neonatal hyperbilirubinemia 1 g per kilogram body weight prior to or during exchange transfusion. (2.1) Adult respiratory distress syndrome (ARDS) Adults: 25 g over 30 minutes and repeated at 8 hours for 3 days, if necessary. (2.1) Prevention of central volume depletion after paracentesis due to cirrhotic ascites Adults: 8 g for every 1000 mL of ascitic fluid removed. (2.1) Do not dilute with sterile water for injection as this may cause hemolysis in recipients. (5.7)
DOSAGE FORMS AND STRENGTHS
Albutein 25% is a solution containing 250 g per L of total protein of which at least 95% is human albumin. (3)
CONTRAINDICATIONS
- Hypersensitivity to albumin preparations or to any of the excipients.
- Severe anemia or cardiac failure with normal or increased intravascular volume. (4)
WARNINGS AND PRECAUTIONS
- Suspicion of allergic or anaphylactic reactions requires immediate discontinuation of the injection and implementation of appropriate medical treatment. (5.1)
- Hypervolemia may occur if the dosage and rate of infusion are not adjusted to the patient's volume status. Use with caution in conditions where hypervolemia and its consequences or hemodilution could represent a special risk to the patient. (5.2)
- When concentrated albumin is administered, care must be taken to assure adequate hydration of the patient. (5.3)
- Monitor electrolytes, coagulation and hematology parameters and hemodynamic status when albumin is administered. (5.4, 5.5, 5.6)
- Do not dilute with sterile water for injection. (5.7)
- This product is made from human plasma and may contain infectious agents, e.g., viruses and, theoretically, the Creutzfeldt-Jakob disease agent. (5.8)
ADVERSE REACTIONS
The most common adverse reactions are anaphylactoid type reactions. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Grifols Biologicals LLC at 1-888-GRIFOLS (1-888-474-3657) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
- Pregnancy: No human or animal data. Use only if clearly needed. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Hypovolemia
1.2 Cardiopulmonary Bypass Procedures (Treatment Adjunct)
1.3 Acute Nephrosis (Treatment Adjunct)
1.4 Hypoalbuminemia
1.5 Ovarian Hyperstimulation Syndrome
1.6 Neonatal hyperbilirubinemia
1.7 Adult Respiratory Distress Syndrome (ARDS) (Treatment Adjunct)
1.8 Prevention of Central Volume Depletion after Paracentesis due to Cirrhotic Ascites (Treatment Adjunct)
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Hypervolemia/Hemodilution
5.3 Dehydration
5.4 Electrolyte Imbalance
5.5 Coagulation Abnormalities
5.6 Laboratory Monitoring
5.7 Application Precautions
5.8 Transmissible Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Hypovolemia
For restoration and maintenance of circulating blood volume where hypovolemia is demonstrated and colloid use is appropriate. When hypovolemia is long standing and hypoalbuminemia exists accompanied by adequate hydration or edema, 20-25% albumin solutions should be used.1,2,3
Acute liver failure is a special situation in which both hypovolemia and hypoalbuminemia can be present. ALBUTEIN 25% can be used in such cases.1
ALBUTEIN 25% may be of value in the treatment of shock or hypotension in renal dialysis patients.1
1.2 Cardiopulmonary Bypass Procedures (Treatment Adjunct)
Preoperative dilution of blood using albumin and crystalloid can be used in cardiopulmonary bypass procedures. Albumin also may be used in the priming fluid.4,5,6
1.3 Acute Nephrosis (Treatment Adjunct)
ALBUTEIN 25% may be used to treat peripheral edema in patients with acute nephrosis who are refractory to cyclophosphamide, corticosteroid therapy or diuretics.1,2,7
1.4 Hypoalbuminemia
ALBUTEIN 25% may be indicated for subjects with hypoalbuminemia who are critically ill and/or actively bleeding. When albumin deficit is the result of excessive protein loss, the effect of ALBUTEIN 25% administration will be temporary unless the underlying disorder is reversed.8,9,10 Septic patients and patients undergoing major surgery may lose more than half of their circulating plasma volume.1,11 Treatment with ALBUTEIN 25% may be of value in such cases, especially when plasma colloid oncotic pressure is abnormally low.1
In the first 24 hours after thermal injury, large volumes of crystalloids are infused to restore the depleted extracellular fluid volume. Beyond 24 hours, ALBUTEIN 25% can be used to maintain plasma colloid osmotic pressure.2,12,13 Protein loss from the third space due to infection (acute peritonitis, pancreatitis, mediastinitis or extensive cellulitis) may require treatment with an infusion of albumin.14,15
1.5 Ovarian Hyperstimulation Syndrome
ALBUTEIN 25% may be used as a plasma volume expander in fluid management relating to severe forms of ovarian hyperstimulation syndrome.16,17
1.6 Neonatal hyperbilirubinemia
ALBUTEIN 25% is indicated for the treatment of neonatal hyperbilirubinemia. It may be used prior to or during an exchange procedure in an attempt to bind free bilirubin and enhance its excretion.18,19,20
-
2 DOSAGE AND ADMINISTRATION
For Intravenous Use Only
2.1 Dosage
Adjust the concentration, dosage and infusion rate of the albumin preparation to the patient's individual requirements.
The dose required depends on the patient's body weight, severity of injury/illness and on continuing fluid and protein losses. Use adequacy of circulating blood volume, not plasma albumin levels, to determine the dose required.
Indication Dose Hypovolemia Adults: Initial dose of 25 g.
If hemodynamic stability is not achieved within 15 to 30 minutes, an additional dose may be given.
Hemodilution may follow administration of Albutein 25%. Anemia resulting from hemorrhage should be corrected by administration of compatible red blood cells or compatible whole blood.
For acute liver failure: initial dose of 12 to 25 g. An infusion rate of 1-2 mL per minute is usually indicated.
For renal dialysis, the initial dose should not exceed 25 g and patients should be carefully observed for signs of fluid overload.Cardiopulmonary bypass procedures Adults: Initial dose of 25 g. Additional amounts may be administered as clinically indicated. Acute nephrosis Adults: 25 g together with diuretic once a day for 7 - 10 days. Hypoalbuminemia Adults: 50 to 75 g
For pre- and post-operative hypoproteinemia: 50 to 75 g.
In burns, therapy usually starts with administration of large volumes of crystalloid solution to maintain plasma volume. After 24 hours: initial dose of 25 g and dose adjustment to maintain plasma protein concentration of 2.5 g per 100 mL or a serum protein concentration of 5.2 g per 100 mL.
Third space protein loss due to infection: initial dose of 50 to 100 g. An infusion rate of 1-2 mL per minute is usually indicated in the absence of shock. Treatment should always be guided by hemodynamic response.Ovarian hyperstimulation syndrome Adults: 50 g to 100 g over 4 hours and repeated at 4-12 hour intervals as necessary, when infusion of normal saline fails to achieve or maintain hemodynamic stability and urine output. Neonatal hyperbilirubinemia 1 g per kilogram body weight prior to or during exchange transfusion. Adult respiratory distress syndrome (ARDS) Adults: 25 g over 30 minutes and repeated at 8 hours for 3 days, if necessary. Prevention of central volume depletion after paracentesis due to cirrhotic ascites Adults: 8 g for every 1000 mL of ascitic fluid removed. 2.2 Administration
Intravenous use only
- ALBUTEIN 25% is a clear and slightly viscous solution. Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the solution is turbid or if there is sediment in the bottle.
- Do not freeze.
- Warm product to room temperature before use if large volumes are administered.
- ALBUTEIN 25% contains no preservatives. Do not begin administration more than 4 hours after the container has been entered. Discard unused portion.
- Do not dilute with sterile water for injection. The product can be diluted in an isotonic solution. (e.g., 5% dextrose in water or 0.9% sodium chloride) [see Warnings and Precautions (5.7)].
- Adjust the infusion rate to the individual circumstances and the indication.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Suspicion of allergic or anaphylactic reactions requires immediate discontinuation of the infusion and implementation of appropriate medical treatment.
5.2 Hypervolemia/Hemodilution
Hypervolemia may occur if the dosage and rate of infusion are not adjusted to the patient's volume status. At the first clinical signs of cardiovascular overload (headache, dyspnea, jugular venous distention, increased blood pressure), the infusion must be slowed or stopped immediately.
Use albumin with caution in conditions where hypervolemia and its consequences or hemodilution could represent a special risk to the patient. Examples of such conditions are:
- Decompensated heart failure
- Hypertension
- Esophageal varices
- Pulmonary edema
- Hemorrhagic diathesis
- Severe anemia
- Renal and post-renal anuria
5.3 Dehydration
The colloid-osmotic effect of human albumin 25% is approximately five times that of blood plasma. Therefore, when concentrated albumin is administered, care must be taken to assure adequate hydration of the patient. Patients should be monitored carefully to guard against circulatory overload and hyperhydration. Patients with marked dehydration require administration of additional fluids.
5.4 Electrolyte Imbalance
20% – 25% human albumin solutions are relatively low in electrolytes compared to 4% – 5% human albumin solutions. Monitor regularly the electrolyte status of the patient and take appropriate steps to restore or maintain the electrolyte balance when albumin is administered.
5.5 Coagulation Abnormalities
Regular monitoring of coagulation and hematology parameters is necessary if comparatively large volumes are to be replaced. Care must be taken to ensure adequate substitution of other blood constituents (coagulation factors, electrolytes, platelets and erythrocytes).
5.6 Laboratory Monitoring
Monitor regularly hemodynamic parameters during administration of ALBUTEIN 25%; this may include:
- Arterial blood pressure and pulse rate
- Central venous pressure
- Pulmonary artery occlusion pressure
- Urine output
- Electrolytes
- Hematocrit/hemoglobin
5.7 Application Precautions
ALBUTEIN 25% must not be diluted with sterile water for injection as this may cause hemolysis in recipients. The product can be diluted in an isotonic solution (e.g., 5% dextrose in water or 0.9% sodium chloride) [see Dosage and Administration (2.2)].
5.8 Transmissible Infectious Agents
Albumin is a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) is also considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for ALBUTEIN 25%.
-
6 ADVERSE REACTIONS
The most serious adverse reactions are anaphylactic shock, heart failure and pulmonary edema.
The most common adverse reactions are anaphylactoid type reactions.
Adverse reactions to Albutein 25% normally resolve when the infusion rate is slowed or the infusion is stopped. In case of severe reactions, the infusion is stopped and appropriate treatment initiated.
6.2 Post-marketing Experience
Because adverse reactions are reported voluntarily post-approval from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to product exposure. The following adverse reactions have been identified during post approval use of human albumin, including ALBUTEIN (all strengths) in decreasing order of significance:
- Anaphylactic shock
- Heart failure
- Pulmonary edema
- Hypotension
- Tachycardia
- Vomiting
- Urticaria
- Rash
- Headache
- Chills
- Fever
- Flushing
- Nausea
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with ALBUTEIN 25%. It is also not known whether ALBUTEIN 25% can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. ALBUTEIN 25% should be given to a pregnant woman only if clearly needed.
-
11 DESCRIPTION
ALBUTEIN 25% is a sterile, aqueous solution for single dose intravenous administration containing 25% human albumin (weight/volume). ALBUTEIN 25% is prepared by a cold alcohol fractionation method from pooled human plasma obtained from venous blood. The product is stabilized with 0.08 millimole sodium caprylate and 0.08 millimole sodium acetyltryptophanate per gram of protein. The colloid osmotic effect of human albumin 25% is approximately five times that of normal human plasma. A liter of ALBUTEIN 25% solution contains 130-160 milliequivalents of sodium ion. The aluminum content of the solution is not more than 200 micrograms per liter during the shelf life of the product. The product contains no preservatives.
ALBUTEIN 25% is manufactured from Source Plasma collected from FDA approved plasmapheresis centers in the United States. ALBUTEIN 25% is heated at 60 °C for ten hours against the possibility of transmitting viruses.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Human Albumin accounts for more than half of the total protein in the plasma and represents about 10% of protein synthesis activity by the liver. Human Albumin 25% has a corresponding hyperoncotic effect.
The primary physiological function of albumin results from its contribution to plasma colloid oncotic pressure and transport function. Albumin stabilizes circulating blood volume and is a carrier of hormones, enzymes, medicinal products and toxins. Other physiological functions include antioxidant properties; free radical scavenging; and capillary membrane integrity.
12.3 Pharmacokinetics
Albumin is distributed throughout the extracellular space and more than 60% of the body albumin pool is located in the extravascular fluid compartment. Albumin has a circulating life span of 15-20 days, with a turnover of approximately 15 g per day.
The balance between synthesis and breakdown is normally achieved by feedback regulation. Elimination is predominantly intracellular and due to lysosome proteases.
In healthy subjects, less than 10% of infused albumin leaves the intravascular compartment during the first 2 hours following infusion. There is considerable individual variation in the effect of albumin on plasma volume. In some patients, the plasma volume can remain elevated for several hours. In critically ill patients, however, albumin can leak out of the vascular space in substantial amounts at an unpredictable rate.
-
15 REFERENCES
- Tullis JL. Albumin: 1. Background and Use. 2. Guidelines for Clinical Use. JAMA. 1977;237:355-360, 460-463.
- Vermeulen LC, et al. A Paradigm for Consensus. Arch Intern Med. 1995;155:373-379.
- SAFE Study investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-2256.
- Sedrakyan A, Gondek K, Paltiel D, et al. Volume expansion with albumin decreases mortality after coronary artery bypass graft surgery. Chest. 2003;123:1853-1857.
- Russell JA, Navickis RJ, Wilkes MM. Albumin versus crystalloid for pump priming in cardiac surgery: meta-analysis of controlled trials. J Cardiothorac Vasc Anesth. 2004;18:429-37.
- American Thoracic Society. Evidence-based colloid use in the critically ill: American Thoracic Society consensus statement. Am J Respir Crit Care Med. 2004;170:1247-59.
- Fliser D, Zurbrüggen I, Mutschler E, et al. Coadministration of albumin and furosemide in patients with nephrotic syndrome. Kidney Int. 1999;55:629-34.
- Mendez CM, McClain CJ, Marsano LS. Albumin Therapy in Clinical Practice. Nutrition in Clinical Practice. 2005;20:314-320.
- Haynes GR, Navickis RJ, Wilkes MM. Albumin administration-what is the evidence of clinical benefit? A systematic review of randomized controlled trials. Eur J Anaesthesiol. 2003 Oct;20(10):771-93.
- Vincent JL, Navickis RJ, Wilkes MM. Morbidity in hospitalized patients receiving human albumin: a meta-analysis of randomized, controlled trials. Crit Care Med. 2004;32:2029-38.
- Skillman JJ, Tanenbaum BJ. Current Topics in Surgical Research. Vol. 2. New York: Academic Press. 1970;523.
- Muir IA, Barclay TL. Burns and their treatment. Chicago: Year Book Medical Publishers. 1974.
- Pruitt BA Jr, Goodwin CW Jr. Current treatment of the extensively burned patient. Surg Annu. 1983;15:331-64.
- Clowes GHA Jr, Vucinic M, Weidner MG. Circulatory and metabolic alterations associated with survival or death in peritonitis: clinical analysis of 25 cases. Ann Surg. 1966;166:866-85.
- Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med.1999;341:403-409.
- Aboulghar M, Evers JH, Al Inany H. Intravenous albumin for preventing severe ovarian hyperstimulation syndrome: a Cochrane review. Hum Reprod. 2002;17:3027-3032.
- Practice Committee of the American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil Steril. 2006;86:S178-S183.
- Tsao YC, Yu VY. Albumin in management of neonatal hyperbilirubinaemia. Arch Dis Child. 1972;47:250-256.
- Practice parameter: management of hyperbilirubinemia in the healthy term newborn. Pediatrics. 1994;94(4 pt 1):558-62.
- Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N Eng J Med. 2001;344:581-90.
- Martin GS, et al. A randomized, controlled trail of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med. 2005; 33:1681-1687.
- Ginés P, Cárdenas A, Arroyo V, et al. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646-54.
- Runyon BA. AASLD Practice Guidelines. Management of adult patients with ascites due to cirrhosis. Hepatology. 2009;49(6):2087-107.
- Moore KP, Wong F, Ginés P, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258-66.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
ALBUTEIN 25% is supplied in single-use, individually laser etched vials.
The following vial sizes of Albutein 25% are available:
NDC Number Fill Size Grams Protein
68516-5216-5 20 mL 5 g
68516-5216-1 50 mL 12.5 g
68516-5216-2 100 mL 25 g
The two larger vial size labels (50 and 100 mL) incorporate integrated hangers. Each label has a peel-off strip showing the product name and lot number.
ALBUTEIN 25% is stable for three years provided the storage temperature does not exceed 30 °C. Protect from freezing.
-
17 PATIENT COUNSELING INFORMATION
This product is usually given in a hospital setting.
Inform patients being treated with ALBUTEIN 25% about the risks and benefits of its use [see Adverse Reactions (6)].
Inform patients to immediately report the following signs and symptoms to their physician:
- Allergic or anaphylactic type reactions [see Warnings and Precautions (5.1)].
- Cardiovascular overload (e.g., headache, dyspnea and jugular venous) [see Warnings and Precautions (5.2)].
- Increased blood pressure, raised venous pressure and pulmonary edema [see Warnings and Precautions (5.2)].
Inform patients that ALBUTEIN 25% is a derivative of human plasma and may contain infectious agents that cause disease (e.g., viruses, and theoretically, the CJD agent). Inform patients that the risk that ALBUTEIN 25% may transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing the donated plasma for certain viral agents and by the inactivation and/or removal of certain viruses during the manufacturing process [see Warnings and Precautions (5.8)].
Manufactured by:
Grifols Biologicals LLC
5555 Valley Boulevard
Los Angeles, CA 90032, U.S.A.
U. S. License No. 1694 -
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 50 mL Vial Label
GRIFOLS
NDC: 68516-5216-3
Albumin (Human) U.S.P.
Albutein® 25%25% 12.5 g 50 mL
Store at temperatures not exceeding 30° C.
DO NOT USE IF TURBID. DO NOT BEGIN ADMINISTRATION MORE
THAN 4 HOURS AFTER THE CONTAINER HAS BEEN ENTERED.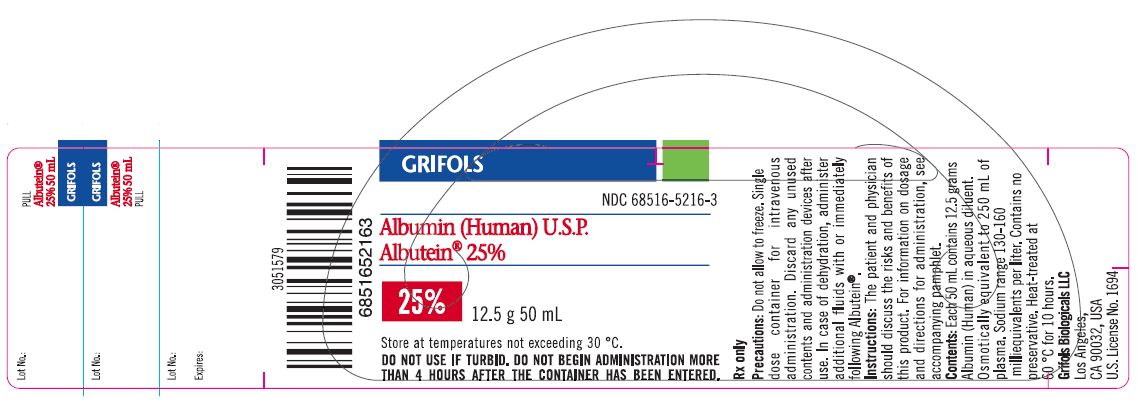
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 50 mL Carton Label
GRIFOLS
NDC: 68516-5216-1
Albumin (Human) U.S.P.
Albutein® 25%Solution 12.5 g 50 mL
25%
Store at temperatures
not exceeding 30° C.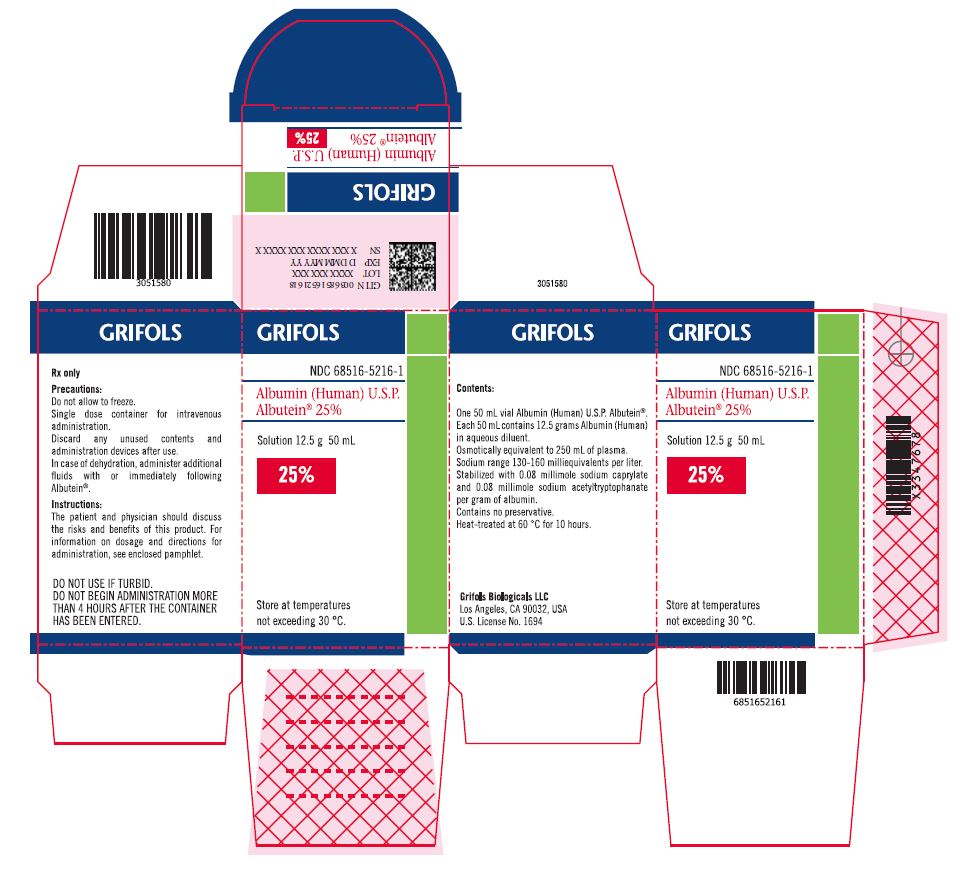
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 100 mL Vial Label
GRIFOLS
NDC: 68516-5216-4
Albumin (Human) U.S.P.
Albutein® 25%25% 25 g 100 mL
Store at temperatures not exceeding 30° C.
DO NOT USE IF TURBID. DO NOT BEGIN ADMINISTRATION MORE
THAN 4 HOURS AFTER THE CONTAINER HAS BEEN ENTERED.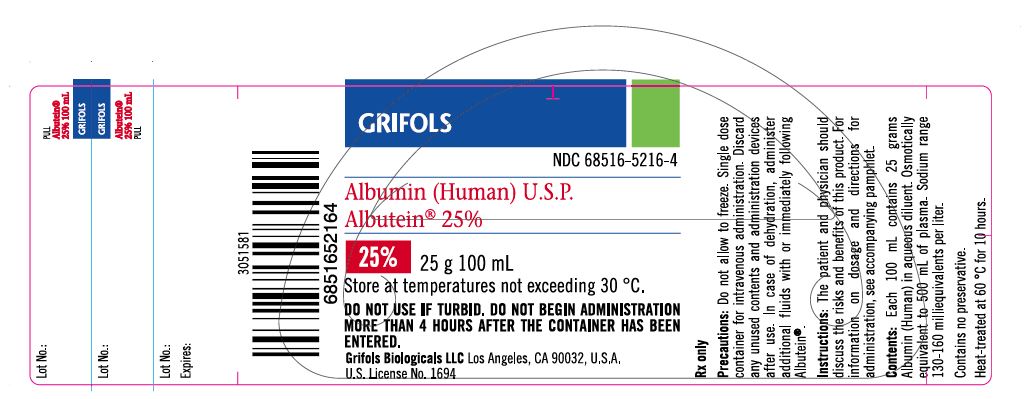
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 100 mL Carton Label
GRIFOLS
NDC: 68516-5216-2
Albumin (Human) U.S.P.
Albutein® 25%Solution 25 g 100 mL
25%
Store at temperatures not exceeding 30° C.

-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 20 mL Vial Label
GRIFOLS
NDC: 68516-5216-6
Albumin (Human) U.S.P.
Albutein® 25%25% 5 g 20 mL
Store at temperatures not exceeding 30° C.
DO NOT USE IF TURBID. DO NOT BEGIN ADMINISTRATION MORE
THAN 4 HOURS AFTER THE CONTAINER HAS BEEN ENTERED.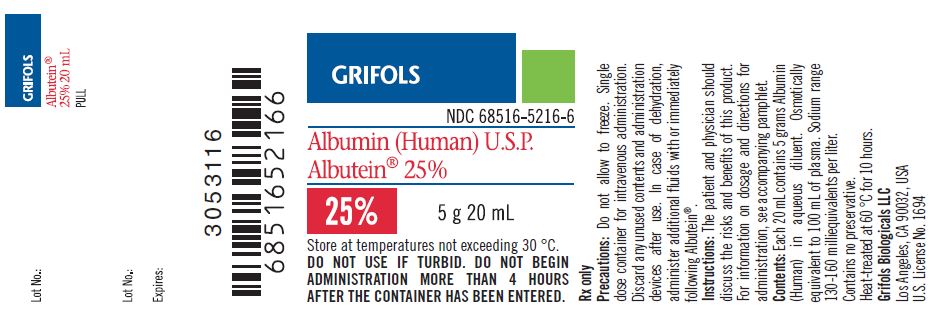
-
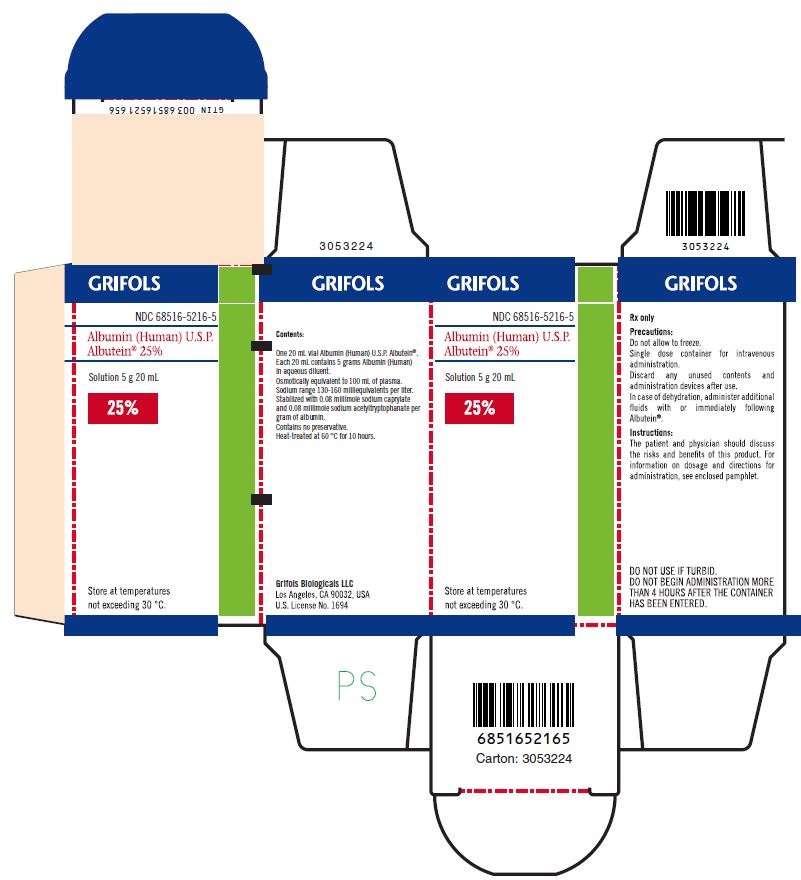
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 20 mL Carton Label
GRIFOLS
NDC: 68516-5216-5
Albumin (Human) U.S.P.
Albutein® 25%Solution 5 g 20 mL
25%
Store at temperatures not exceeding 30° C.
-
INGREDIENTS AND APPEARANCE
ALBUTEIN
albumin (human) injection, solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 68516-5216 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Albumin Human (UNII: ZIF514RVZR) (Albumin Human - UNII:ZIF514RVZR) Albumin Human 12.5 g in 50 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) Sodium Caprylate (UNII: 9XTM81VK2B) Sodium Acetyltryptophanate (UNII: 3EN9H0M2FX) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68516-5216-1 1 in 1 CARTON 1 NDC: 68516-5216-3 50 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 68516-5216-2 1 in 1 CARTON 2 NDC: 68516-5216-4 100 mL in 1 VIAL; Type 0: Not a Combination Product 3 NDC: 68516-5216-5 1 in 1 CARTON 3 NDC: 68516-5216-6 20 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA102478 08/15/1978 Labeler - GRIFOLS USA, LLC (048987452) Establishment Name Address ID/FEI Business Operations Grifols Biologicals LLC 092694538 manufacture(68516-5216) Establishment Name Address ID/FEI Business Operations Grifols Biologicals LLC 121076871 manufacture(68516-5216) Establishment Name Address ID/FEI Business Operations Grifols Therapeutics LLC 611019113 manufacture(68516-5216) Establishment Name Address ID/FEI Business Operations INSTITUTO GRIFOLS SA 465562213 manufacture(68516-5216)
Trademark Results [ALBUTEIN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALBUTEIN 72417224 0964012 Live/Registered |
ABBOTT LABORATORIES 1972-03-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.