Lab 52 Essential Oil Sensitive by TOOTHFILM INC Marketing End 003
Lab 52 Essential Oil Sensitive by
Drug Labeling and Warnings
Lab 52 Essential Oil Sensitive by is a Otc medication manufactured, distributed, or labeled by TOOTHFILM INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
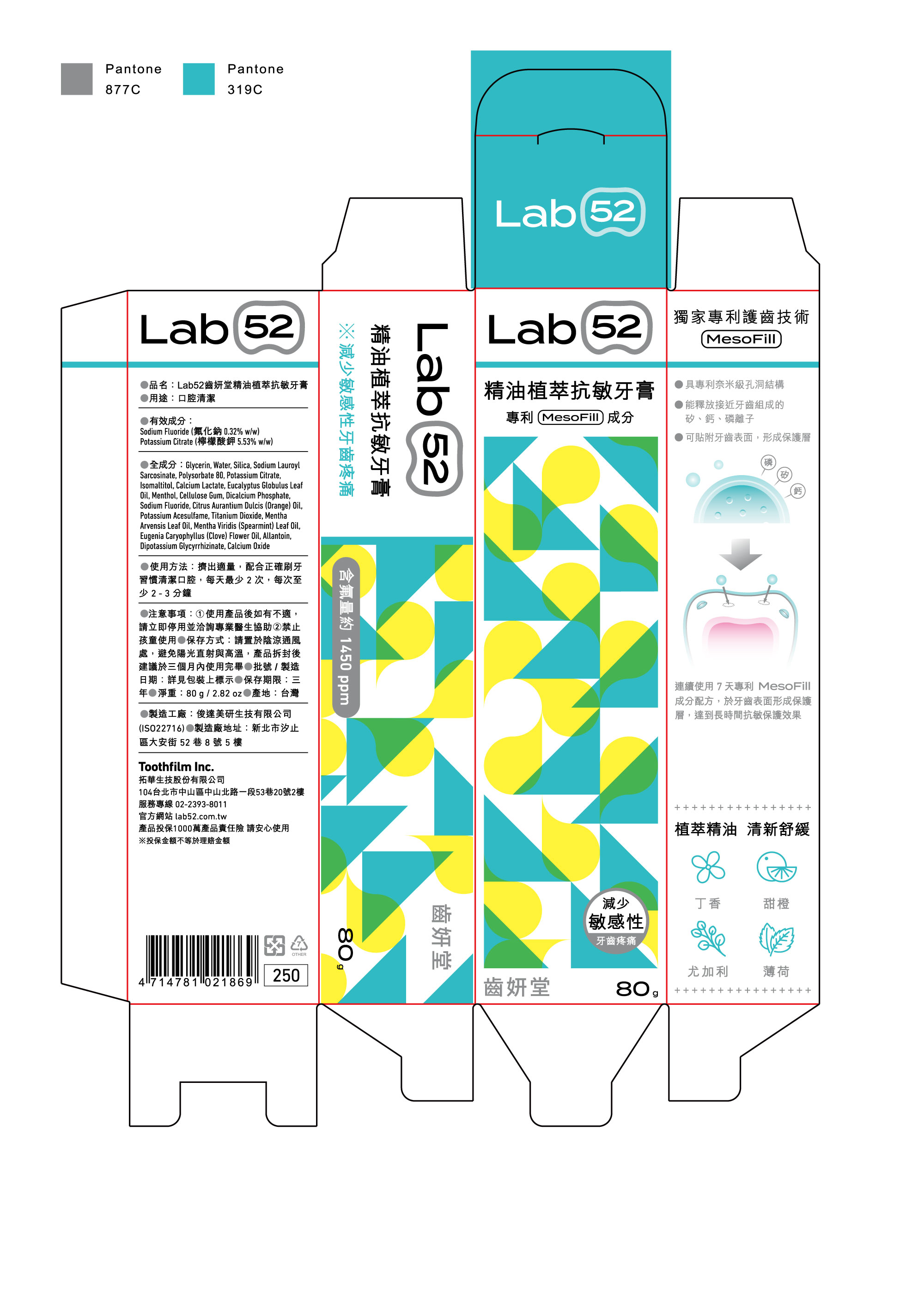
LAB 52 ESSENTIAL OIL SENSITIVE- potassium citrate, sodium fluoride paste, dentifrice
TOOTHFILM INC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Marketing End 003
Glycerin, Water, Silica, Sodium Lauroyl Sarcosinate, Polysorbate 80, Isomaltitol, Calcium Lactate, Eucalyptus Globulus Leaf Oil, Menthol, Cellulose Gum, Dicalcium Phosphate, Citrus Aurantium Dulcis (Orange) Oil, Potassium Acesulfame, Titanium Dioxide, Mentha Arvensis Leaf Oil, Mentha Viridis (Spearmint) Leaf Oil, Eugenia Caryophyllus (Clove) Flower Oil, Allantoin, Dipotassium Glycyrrhizinate, Calcium Oxide
Squeeze out an appropriate amount and clean your mouth with correct brushing habits, at least 2 times a day, at least 2-3 minutes each time
If you feel uncomfortable after using the product, please stop using it immediately and consult a professional doctor for assistance
| LAB 52 ESSENTIAL OIL SENSITIVE
potassium citrate, sodium fluoride paste, dentifrice |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - TOOTHFILM INC (656036549) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TOOTHFILM INC | 656036549 | manufacture(82711-003) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.