Kids Anticavity fluoride by TOOTHFILM INC Marketing End 005
Kids Anticavity fluoride by
Drug Labeling and Warnings
Kids Anticavity fluoride by is a Otc medication manufactured, distributed, or labeled by TOOTHFILM INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
KIDS ANTICAVITY FLUORIDE- sodium fluoride paste, dentifrice
TOOTHFILM INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Marketing End 005
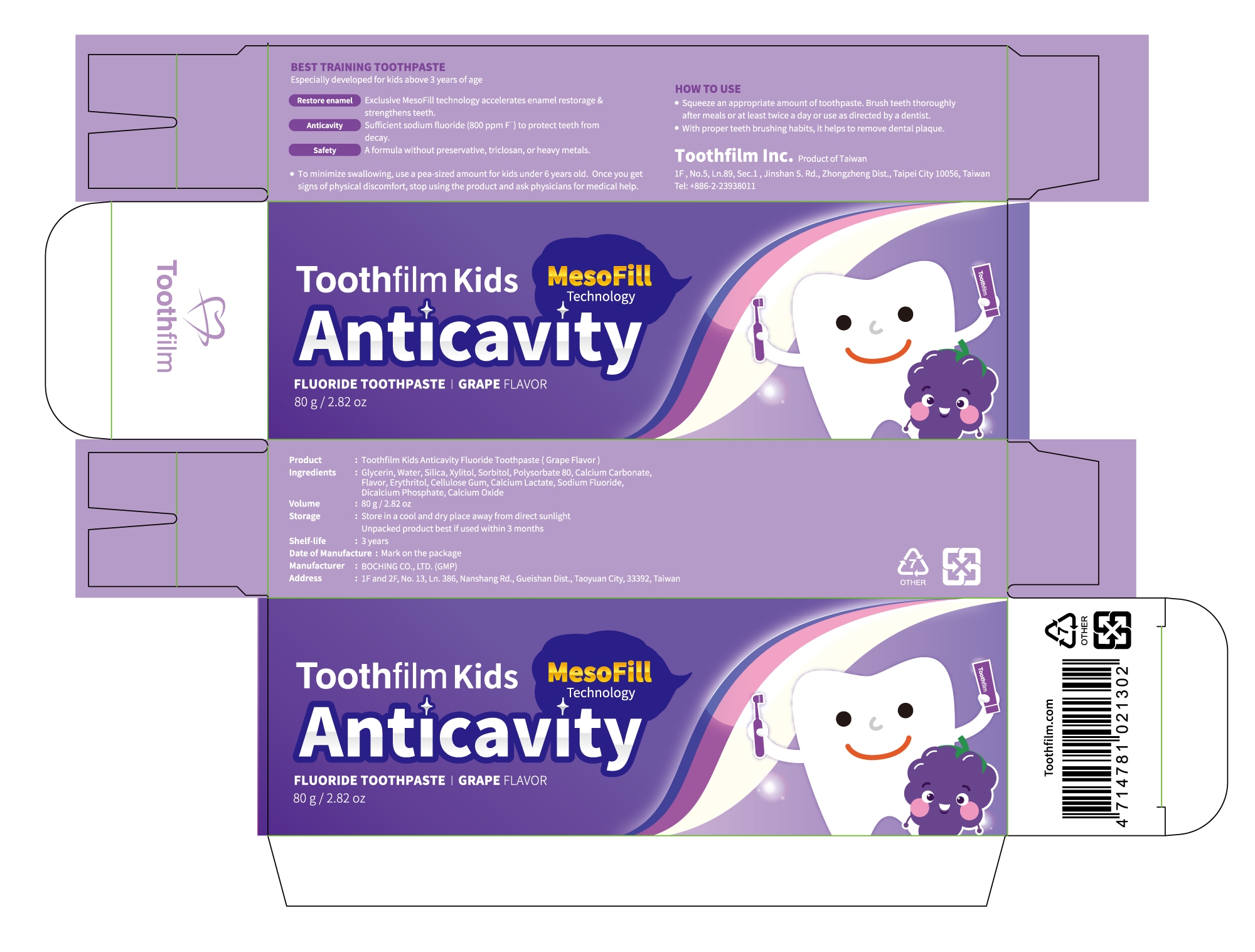
Kids Anticavity Fluoride Toothpaste(Grape)
BEST TRAINING TOOTHPASTE
Especially developed for kids above 3 years of age
A formula without preservative, triclosan, or heavy metals.
Glycerin, Water, Silica, Xylitol, Sorbitol, Polysorbate 80, Isomaltitol, Calcium Carbonate, Grape Flavor, Erythritol, Cellulose Gum, Calcium Lactate, Potassium Acesulfame, Dicalcium Phosphate, Calcium Oxide
. Squeeze an appropriate amount of toothpaste.Brush teeth thoroughlyafter meals or at least twice a day or use as directed by a dentist.
.To minimize swallowing, use a pea-sized amount for kids under 6 years old.Once you get signs of physical discomfort, stop using the product and ask physicians for medical help.
. Squeeze an appropriate amount of toothpaste.Brush teeth thoroughlyafter meals or at least twice a day or use as directed by a dentist.
. With proper teeth brushing habits, it helps to remove dental plaque.
Please keep out of reach of children under 6 years old.
Children under six years of age must be supervised by an adult
Restore enamel
Exclusive MesoFill technology accelerates enamel restorage & strengthens teeth.
Anticavity
Sufficient sodium fluoride (800 ppm F ) to protect teeth from decay.
| KIDS ANTICAVITY FLUORIDE
sodium fluoride paste, dentifrice |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - TOOTHFILM INC (656036549) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TOOTHFILM INC | 656036549 | manufacture(82711-005) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.