AXIM Allergy by VIVUNT PHARMA LLC Axim Allergy
AXIM Allergy by
Drug Labeling and Warnings
AXIM Allergy by is a Otc medication manufactured, distributed, or labeled by VIVUNT PHARMA LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
AXIM ALLERGY- diphenhydramine hydrochloride capsule, liquid filledÂ
VIVUNT LLC
----------
Axim Allergy
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Directions
- Take every 4 to 6 hours, or as directed by a doctor
- Do not take more than 12 Softgels in 24 hours for Adults and do not take more than 6 Softgels for children 6 to 12 years of age in 24 hours.
| adults and children 12 years and over | 1 or 2 Softgels |
| children 6 to under 12 years | 1 Softgel |
| children under 6 years | do not use |
Other information
- Store at 20° - 25 °C (68 °- 77 °F)
- Read all product information before using
- Tamper Evident: Do not use if the foil printed on the blister is torn or ripped.
Inactive ingredients
Gelatin, Glycerin, Methylparaben, Polyethylene Glycol 400, Propylparaben, Purified Water, Sorbitol, Titanium Dioxide
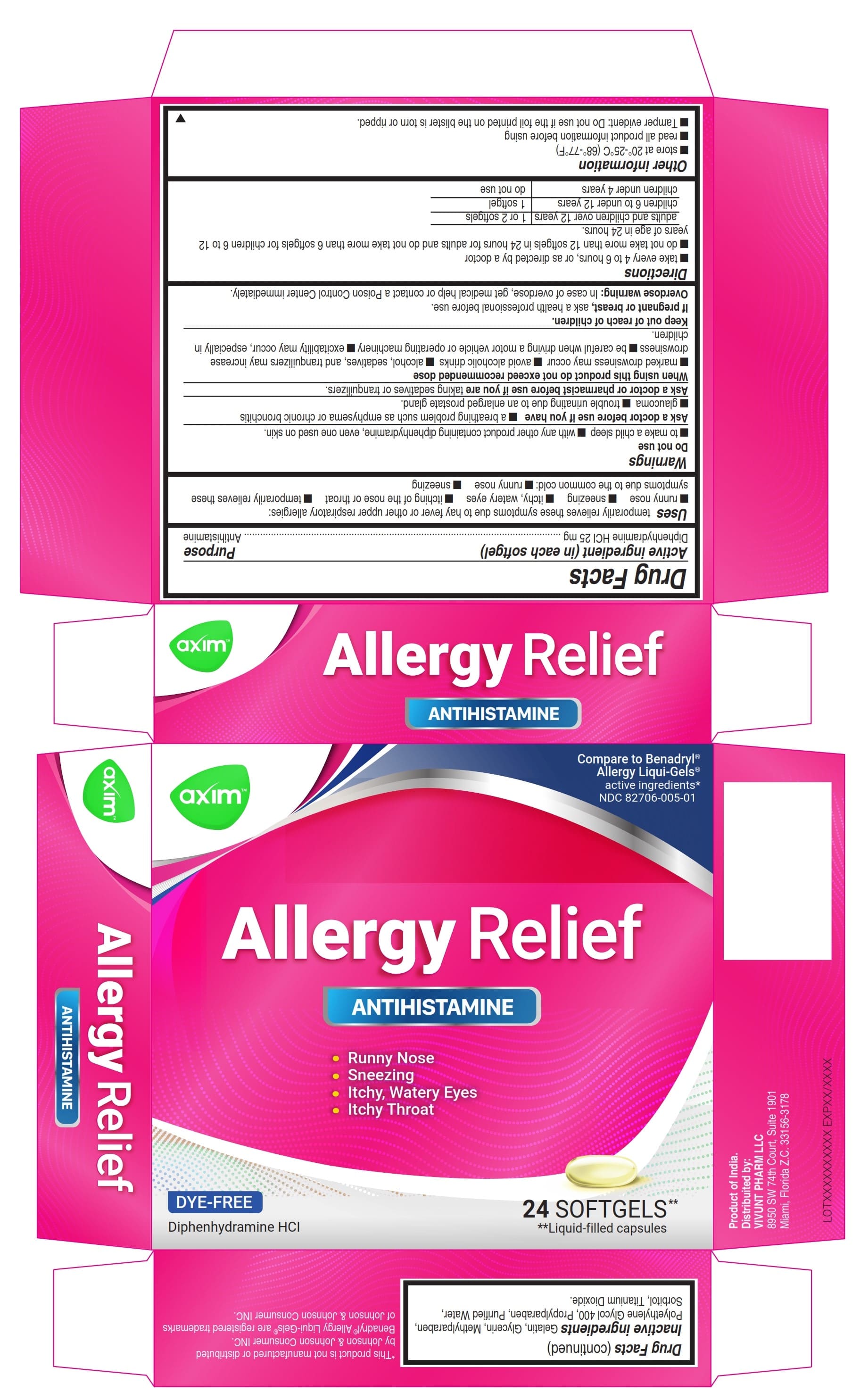
PRINCIPAL DISPLAY PANEL 24
Compare to Benadryl® Allergy Liqui-Gels®
active ingredients*
NDC: 82706-005-01
Antihistamine
- Runny Nose
- Sneezing
- Itchy, Watery Eyes
- Itchy Throat
DYE-FREE
Diphenhydramine HCl
24 SOFTGELS** **Liquid-filled capsules
PRINCIPAL DISPLAY PANEL 6
Compare to Benadryl® Allergy Liqui-Gels®
active ingredients*
NDC: 82706-005-02
Antihistamine
Runny Nose
Sneezing
Itchy, Watery Eyes
Itchy Throat
DYE-FREE
Diphenhydramine HCl
6 SOFTGELS** **Liquid-filled capsules

| AXIM ALLERGYÂ
diphenhydramine hydrochloride capsule, liquid filled |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler -Â VIVUNT LLC (045829437) |
Revised: 8/2025
Â
Document Id: 3ccdebd7-941d-6538-e063-6394a90a9a3f
Set id: de9eb2bf-7c32-558e-e053-2995a90a5c83
Version: 11
Effective Time: 20250820
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.