NATESTO NASAL GEL- testosterone gel, metered
Natesto by
Drug Labeling and Warnings
Natesto by is a Prescription medication manufactured, distributed, or labeled by Acerus Pharmaceuticals Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NATESTO safely and effectively. See full prescribing information for NATESTO.
Natesto (testosterone) nasal gel CIII Initial U.S. Approval: 1953RECENT MAJOR CHANGES
Warnings and Precautions ( 5.7) 10/2016 INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Prior to initiating Natesto, confirm the diagnosis of hypogonadism by ensuring that serum testosterone has been measured in the morning on at least two separate days and that these concentrations are below the normal range ( 2).
- The recommended dose of Natesto is 11 mg of testosterone (2 pump actuations, one per nostril), applied intranasally three times daily. ( 2.1)
- Serum total testosterone concentrations should be checked periodically, starting as soon as one month after initiating treatment with Natesto. If total testosterone concentrations consistently exceed 1050 ng/dL, therapy with Natesto should be discontinued. If total testosterone concentrations are consistently below 300 ng/dL, an alternative treatment should be considered. ( 2.1)
- Not recommended for use with nasally administered drugs other than sympathomimetic decongestants (e.g., oxymetazoline). ( 2.3, 7.4, 12.3).
DOSAGE FORMS AND STRENGTHS
Natesto nasal gel is available as a metered-dose pump. One pump actuation delivers 5.5 mg of testosterone. ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Nasal adverse reactions: Instruct patients to report any nasal symptoms or signs ( 5.1)
- Not recommended for use in patients with chronic nasal conditions or alterations in nasal anatomy. ( 5.2)
- Monitor patients with benign prostatic hyperplasia (BPH) for worsening of signs and symptoms of BPH. ( 5.3)
- Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients using testosterone products. Evaluate patients with signs or symptoms consistent with DVT or PE. ( 5.5)
- Some postmarketing studies have shown an increased risk of myocardial infarction and stroke associated with use of testosterone replacement therapy. ( 5.6)
- Testosterone has been subject to abuse, typically at doses higher than recommended for the approved indication and in combination with other anabolic androgenic steroids. ( 5.7)
- Women and children should not use Natesto. ( 5.8)
- Exogenous administration of androgens may lead to azoospermia ( 5.9)
- Edema with or without congestive heart failure (CHF) may be a complication in patients with preexisting cardiac, renal, or hepatic disease. ( 5.11)
- Sleep apnea may occur in those with risk factors. ( 5.13)
- Monitor prostate-specific antigen (PSA), hematocrit and lipid concentrations periodically. ( 5.3, 5.4, 5.14)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥3%) are: PSA increased, headache, rhinorrhea, epistaxis, nasal discomfort, nasopharyngitis, bronchitis, upper respiratory tract infection, sinusitis, and nasal scab. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Aytu Bioscience at 1-855-298-8246 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Androgens may decrease blood glucose and therefore may decrease insulin requirements in diabetic patients. ( 7.1)
- Changes in anticoagulant activity may be seen with androgens. More frequent monitoring of International Normalized Ratio (INR) and prothrombin time is recommended in patients taking warfarin. ( 7.2)
- Use of testosterone with corticosteroids may result in increased fluid retention. Use with caution, particularly in patients with cardiac, renal, or hepatic disease. ( 7.3)
USE IN SPECIFIC POPULATIONS
There are insufficient long-term safety data in geriatric patients using Natesto to assess the potential risks of cardiovascular disease and prostate cancer. ( 8.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1. Dosing
2.2. Administration Instructions
2.3. Use with Nasally Administered Drugs Other Than Sympathomimetic Decongestants
2.4. Temporary Discontinuation of Use for Severe Rhinitis
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Nasal Adverse Reactions and Limited Long-Term Information on Nasal Safety
5.2 Use in Patients with Chronic Nasal Conditions and Alterations in Nasal Anatomy
5.3 Worsening of Benign Prostatic Hyperplasia and Potential Risk of Prostate Cancer
5.4 Polycythemia
5.5 Venous Thromboembolism
5.6 Cardiovascular Risk
5.7 Abuse of Testosterone and Monitoring of Serum Testosterone Concentrations
5.8 Use in Women
5.9 Potential for Adverse Effects on Spermatogenesis
5.10 Hepatic Adverse Effects
5.11 Edema
5.12 Gynecomastia
5.13 Sleep Apnea
5.14 Lipids
5.15 Hypercalcemia
5.16 Decreased Thyroxine-binding Globulin
6. ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7. DRUG INTERACTIONS
7.1 Insulin
7.2 Oral Anticoagulants
7.3 Corticosteroids
7.4 Oxymetazoline
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
8.3. Nursing Mothers
8.4. Pediatric Use
8.5. Geriatric Use
8.6. Renal Impairment
8.7. Hepatic Impairment
8.8. Use in Men With Body Mass Index greater than 35 kg/m 2
8.9. Allergic Rhinitis
9. DRUG ABUSE AND DEPENDENCE
9.1. Controlled Substance
9.2. Abuse
9.3. Dependence
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
12.2. Pharmacodynamics
12.3. Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, and Impairment of Fertility
14. CLINICAL STUDIES
14.1. Testosterone Replacement Therapy
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. How Supplied
16.2. Storage
16.3. Handling and Disposal
17. PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1. INDICATIONS AND USAGE
Natesto is indicated for replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone.
- Primary hypogonadism (congenital or acquired): testicular failure due to conditions such as cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter's syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations and gonadotropins (follicle-stimulating hormone [FSH], luteinizing hormone [LH]) above the normal range.
- Hypogonadotropic hypogonadism (congenital or acquired): gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum concentrations but have gonadotropins in the normal or low range.
Limitations of use:
- Safety and efficacy of Natesto in men with “age-related hypogonadism” (also referred to as “late-onset hypogonadism”) have not been established.
- Safety and efficacy of Natesto in males less than 18 years old have not been established [see Use in Specific Populations (8.4)].
-
2. DOSAGE AND ADMINISTRATION
Prior to initiating Natesto, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these serum testosterone concentrations are below the normal range.
2.1. Dosing
The recommended dose of Natesto is 11 mg of testosterone (2 pump actuations; 1 actuation per nostril) administered intranasally three times daily for a total daily dose of 33 mg.
Serum total testosterone concentrations should be checked periodically, starting as soon as one month after initiating treatment with Natesto. When the total testosterone concentration consistently exceeds 1050 ng/dL, therapy with Natesto should be discontinued. If the total testosterone concentration is consistently below 300 ng/dL, an alternative treatment should be considered.
2.2. Administration Instructions
Natesto is administered intranasally three times daily once in the morning, once in the afternoon and once in the evening (6 to 8 hours apart), preferably at the same time each day. Patients should be instructed to completely depress the pump 1 time in each nostril to receive the total dose. Do not administer Natesto to other parts of the body.
Preparing the Pump
When using Natesto for the first time, patients should be instructed to prime the pump by inverting the pump, depressing the pump 10 times, and discarding any small amount of product dispensed directly into a sink and then washing the gel away thoroughly with warm water. The tip should be wiped with a clean, dry tissue. If the patient gets Natesto gel on their hands, it is recommended that they wash their hands with warm water and soap. This priming should be done only prior to the first use of each dispenser.
Administering the Dose
To administer the dose, patients should be instructed to perform the following steps:
- Blow the nose.
- Remove the cap from the dispenser.
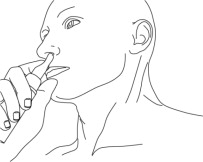
- Place the right index finger on the pump of the actuator and while in front of a mirror, slowly advance the tip of the actuator into the left nostril upwards until their finger on the pump reaches the base of the nose.
- Tilt the actuator so that the opening on the tip of the actuator is in contact with the lateral wall of the nostril to ensure that the gel is applied to the nasal wall.
- Slowly depress the pump until it stops.
- Remove the actuator from the nose while wiping the tip along the inside of the lateral nostril wall to fully transfer the gel.
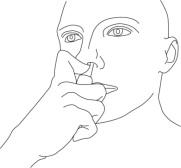
- Using your left index finger, repeat the steps outlined in bullets 3 through 6 for the right nostril.
- Use a clean, dry tissue to wipe the tip of the actuator.
- Replace the cap on the dispenser.
- Press on the nostrils at a point just below the bridge of the nose and lightly massage.
- Refrain from blowing the nose or sniffing for 1 hour after administration.
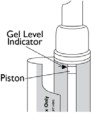
The dispenser should be replaced when the top of the piston inside the dispenser reaches the arrow at the top of the inside label. The inside label may be found by unwrapping the outer flap from around the container.
2.3. Use with Nasally Administered Drugs Other Than Sympathomimetic Decongestants
The drug interaction potential between Natesto and nasally administered drugs other than sympathomimetic decongestants is unknown. Therefore, Natesto is not recommended for use with nasally administered drugs other than sympathomimetic decongestants
(e.g., oxymetazoline) [see Drug Interactions (7.4) and Clinical Pharmacology (12.3)].
2.4. Temporary Discontinuation of Use for Severe Rhinitis
If the patient experiences an episode of severe rhinitis, temporarily discontinue Natesto therapy pending resolution of the severe rhinitis symptoms. If the severe rhinitis symptoms persist, an alternative testosterone replacement therapy is recommended.
- 3. DOSAGE FORMS AND STRENGTHS
-
4. CONTRAINDICATIONS
Natesto is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate [ see Warnings and Precaution (5.3)].
Natesto is contraindicated in women who are or who may become pregnant, or who are breast- feeding. Natesto may cause fetal harm when administered to a pregnant woman. Natesto may cause serious adverse reactions in nursing infants. Exposure of a fetus or nursing infant to androgens may result in varying degrees of virilization. If a pregnant woman is exposed to Natesto, she should be apprised of the potential hazard to the fetus [ see Warnings and Precautions (5.8) and Use in Specific Populations ( 8.1, 8.3)].
-
5. WARNINGS AND PRECAUTIONS
5.1 Nasal Adverse Reactions and Limited Long-Term Information on Nasal Safety
Nasal adverse reactions, including nasopharyngitis, rhinorrhea, epistaxis, nasal discomfort and nasal scabbing, were reported in the clinical trial experience with Natesto. All nasal adverse reactions except one (a single case of upper respiratory infection) were reported as mild or moderate in severity; however, long-term clinical trial data on nasal safety is available in a limited number of subjects [ see Adverse Reactions (6.2)]. Patients should be instructed to report any nasal symptoms or signs to their health care professional. In that circumstance, health care professionals should determine whether further evaluation (e.g., otorhinolaryngology consultation) or discontinuation of Natesto is appropriate.
5.2 Use in Patients with Chronic Nasal Conditions and Alterations in Nasal Anatomy
Due to lack of clinical data on the safety or efficacy, Natesto is not recommended for use in the following patients:
- History of nasal disorders;
- History of nasal or sinus surgery;
- History of nasal fracture within the previous 6 months or nasal fracture that caused a deviated anterior nasal septum;
- Mucosal inflammatory disorders (e.g, Sjogren's syndrome); and
- Sinus disease.
5.3 Worsening of Benign Prostatic Hyperplasia and Potential Risk of Prostate Cancer
- Patients with BPH treated with androgens are at an increased risk for worsening of signs and symptoms of BPH. Monitor patients with BPH for worsening signs and symptoms.
- Patients treated with androgens may be at increased risk for prostate cancer. Evaluate patients for prostate cancer prior to initiating treatment. It would be appropriate to re-evaluate patients 3 to 6 months after initiation of treatment and then in accordance with prostate cancer screening practices [ see Contraindications (4)].
5.4 Polycythemia
Increases in hematocrit, reflective of increases in red blood cell mass, may require discontinuation of Natesto. Check hematocrit prior to initiating testosterone treatment. It would be appropriate to re-evaluate the hematocrit 3 to 6 months after starting testosterone treatment, and then annually. If hematocrit becomes elevated, stop therapy until hematocrit decreases to an acceptable level. An increase in red blood cell mass may increase the risk of thromboembolic events.
5.5 Venous Thromboembolism
There have been postmarketing reports of venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), in patients using testosterone products such as Natesto. Evaluate patients who report symptoms of pain, edema, warmth and erythema in the lower extremity for (DVT) and those who present with acute shortness of breath for PE. If a venous thromboembolic event is suspected, discontinue treatment with Natesto and initiate appropriate workup and management [see Adverse Reactions (6.2)].
5.6 Cardiovascular Risk
Long term clinical safety trials have not been conducted to assess the cardiovascular outcomes of testosterone replacement therapy in men. To date, epidemiologic studies and randomized controlled trials have been inconclusive for determining the risk of major adverse cardiovascular events (MACE), such as non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, with the use of testosterone compared to non-use. Some studies, but not all, have reported an increased risk of MACE in association with use of testosterone replacement therapy in men. Patients should be informed of this possible risk when deciding whether to use or to continue to use Natesto.
5.7 Abuse of Testosterone and Monitoring of Serum Testosterone Concentrations
Testosterone has been subject to abuse, typically at doses higher than recommended for the approved indication and in combination with other anabolic androgenic steroids. Anabolic androgenic steroid abuse can lead to serious cardiovascular and psychiatric adverse reactions [see Drug Abuse and Dependence ( 9)].
If testosterone abuse is suspected, check serum testosterone concentrations to ensure they are within therapeutic range. However, testosterone levels may be in the normal or subnormal range in men abusing synthetic testosterone derivatives. Counsel patients concerning the serious adverse reactions associated with abuse of testosterone and anabolic androgenic steroids. Conversely, consider the possibility of testosterone and anabolic androgenic steroid abuse in suspected patients who present with serious cardiovascular or psychiatric adverse events.
5.8 Use in Women
Due to lack of controlled studies in women and potential virilizing effects, Natesto is not indicated for use in women.
5.9 Potential for Adverse Effects on Spermatogenesis
With large doses of exogenous androgens, including Natesto, spermatogenesis may be suppressed through feedback inhibition of pituitary follicle-stimulating hormone (FSH), which could possibly lead to adverse effects on semen parameters, including sperm count.
5.10 Hepatic Adverse Effects
Prolonged use of high doses of orally active 17-alpha-alkyl androgens (methyltestosterone) has been associated with serious hepatic adverse effects (peliosis hepatitis, hepatic neoplasms, cholestatic hepatitis, and jaundice). Peliosis hepatitis can be a life-threatening or fatal complication. Long-term therapy with intramuscular testosterone enanthate has produced multiple hepatic adenomas. Natesto is not known to cause these adverse effects. Nonetheless, patients should be instructed to report any signs or symptoms of hepatic dysfunction (e.g., jaundice). If these occur, promptly discontinue Natesto while the cause is evaluated.
5.11 Edema
Androgens, including Natesto, may promote retention of sodium and water. Edema, with or without congestive heart failure, may be a serious complication in patients with pre-existing cardiac, renal, or hepatic disease. In addition to discontinuation of the drug, diuretic therapy may be required.
5.12 Gynecomastia
Gynecomastia may develop and may persist in patients being treated with androgens, including Natesto, for hypogonadism .[see Adverse Reactions ( 6.1)].
5.13 Sleep Apnea
The treatment of hypogonadal men with testosterone may potentiate sleep apnea in some patients, especially those with risk factors such as obesity and chronic lung disease.
5.14 Lipids
Changes in the serum lipid profile may occur. Monitor the lipid profile periodically, particularly after starting testosterone therapy. Changes in serum lipid profile may require discontinuation of testosterone therapy.
5.15 Hypercalcemia
Androgens, including Natesto, should be used with caution in cancer patients at risk of hypercalcemia (and associated hypercalciuria). Regular monitoring of serum calcium concentrations is recommended in these patients.
5.16 Decreased Thyroxine-binding Globulin
Androgens, including Natesto, may decrease concentrations of thyroxine-binding globulins, resulting in decreased total T4 serum concentrations and increased resin uptake of T3 and T4. Free thyroid hormone concentrations remain unchanged; however, and there is no clinical evidence of thyroid dysfunction.
-
6. ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Natesto was evaluated in a multicenter, open-label, 90-day clinical study. Patients could continue treatment with Natesto in two, open-label extension periods for an additional 90 and 180 days, respectively. A total of 306 hypogonadal men with morning testosterone concentrations ≤ 300 ng/dL received Natesto. Of these, 78 received Natesto at a dose of 11 mg three times daily.
90-Day Clinical Study
Among the 78 patients who received Natesto three times daily in the 90-day clinical study, the most common adverse reactions were: prostate specific antigen (PSA) increased, headache, rhinorrhea, epistaxis, nasal discomfort, nasopharyngitis, upper respiratory tract infection (URI), sinusitis, bronchitis and nasal scab. PSA increased was considered an adverse reaction by meeting one of two pre-specified criteria: (1) increase from baseline serum PSA greater than 1.4 ug/L, or (2) serum PSA greater than 4.0 ug/L.
Table 1 shows adverse reactions reported by ≥3% of patients treated with 11 mg three times daily in the 90-day clinical study.
Table 1: Adverse Reactions Reported by ≥3% of Patients Treated with Natesto (11 mg of testosterone) Three Times Daily in the 90-Day Clinical Study Adverse Reactions Natesto
(11 mg of Testosterone) Three Times Daily
(N=78)
n (%)PSA increased 4 (5.1) Headache 3 (3.8) Rhinorrhea 3 (3.8) Epistaxis 3 (3.8) Nasal discomfort 3 (3.8) Nasopharyngitis 3 (3.8) Bronchitis 3 (3.8) Upper respiratory tract infection 3 (3.8) Sinusitis 3 (3.8) Nasal scab 3 (3.8) Adverse reactions reported by >2% but <3% of patients in the 90-day clinical study include: blood pressure increased, dysgeusia, nasal dryness, nasal congestion, and cough.
Extension Periods
Among the 78 patients who received Natesto three times daily in the 90-day clinical study, a total of 69 patients received Natesto three times daily in the first 90-day extension period.
Among these 69 patients, the most common adverse reactions were: nasopharyngitis, PSA increased, parosmia, nasal discomfort, rhinorrhea and nasal scab.
Table 2 shows adverse reactions reported by ≥3% of patients who received Natesto three times daily in both the 90-day clinical study and in the 90-day extension period.
Table 2: Adverse Reactions Reported by ≥3% of Patients in Both the 90-Day Clinical Study and in the 90-Day Extension Period Adverse Reactions Natesto 11 mg TID
(N=69)
n (%)Nasopharyngitis 6 (8.7) Rhinorrhea 5 (7.2) PSA increased 4 (5.8) Parosmia 4 (5.8) Nasal discomfort 4 (5.8) Nasal Scab 4 (5.8) Upper respiratory tract infection 3 (4.3) Bronchitis 3 (4.3) Procedural pain 3 (4.3) Pain in extremity 3 (4.3) Headache 3 (4.3) Epistaxis 3 (4.3) A total of 18 patients received Natesto three times daily in all three treatment periods, including the 90-day clinical study, the first 90-day extension period, and the second 180-day extension period. Among these 18 patients, the following adverse reactions were reported in more than one patient each: nasopharyngitis, parosmia, PSA increased, nasal discomfort, nasal scab and hypertension. The following adverse reactions were reported in one patient each: nausea, nasal excoriation, thyroid stimulating hormone increased, decreased appetite, myalgia, anosmia, testicular atrophy, epistaxis, nasal septum disorder, nasal discomfort, and rhinorrhea.
In patients who received Natesto three times daily, mean serum PSA concentrations increased by 0.2 ng/mL, 0.1 ng/mL, and 0.2 ng/mL after 90, 180 and 360 days, respectively.
Discontinuations due to Adverse Reactions
Among all subjects (n=306) who received Natesto at any dose in the 90-day clinical study and its 90- and 180-day extension periods, a total of 6 subjects withdrew from treatment for the following adverse reactions, reported by 1 subject each: nasal discomfort, headache, dysgeusia, PSA increased, allergic reaction (hives, swollen lips and tongue), and 1 patient with myalgia, arthralgia, fever, chills and petechiae.
Increased Hematocrit
Among all subjects (n=306) who received Natesto at any dose in the 90-day clinical study and its 90- and 180-day extension periods, a total of 4 subjects had a hematocrit level > 55%. These 4 patients had baseline hematocrits of 48% and 51%. In no case did hematocrit exceed 58%.
Nasal Adverse Reactions
Among all subjects (n=306) who received Natesto at any dose in the 90-day clinical study and its 90- and 180-day extension periods, the following nasal adverse reactions were reported: nasopharyngitis (8.2%), rhinorrhea (7.8%), epistaxis (6.5%), nasal discomfort (5.9%), parosmia (5.2%), nasal scab (5.2%), upper respiratory infection (4.2%), nasal dryness (4.2%), and nasal congestion (3.9%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of testosterone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular Disorders: myocardial infarction, stroke [see Warnings and Precautions ( 5.6)]
Vascular Disorders: Venous thromboembolism [see Warnings and Precautions (5.5)]
-
7. DRUG INTERACTIONS
7.1 Insulin
Changes in insulin sensitivity or glycemic control may occur in patients treated with androgens. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and, therefore, may necessitate a decrease in the dose of anti-diabetic medication.
7.2 Oral Anticoagulants
Changes in anticoagulant activity may be seen with androgens, therefore more frequent monitoring of international normalized ration (INR) and prothrombin time is recommended in patients taking warfarin, especially at the initiation and termination of androgen therapy.
7.3 Corticosteroids
The concurrent use of testosterone with corticosteroids may result in increased fluid retention and requires monitoring particularly in patients with cardiac, renal, or hepatic disease.
7.4 Oxymetazoline
A 2.6% decrease in mean AUC(0-24) and 3.6% decrease in mean C max of total testosterone was observed in males with symptomatic seasonal rhinitis when treated with oxymetazoline 30 minutes prior to Natesto compared to when left untreated. Oxymetazoline does not impact the absorption of testosterone when concomitantly administered with Natesto [see Clinical Pharmacology (12.3)]. Drug interaction potential with other nasally administered drugs other than oxymetazoline has not been studied.
-
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Pregnancy Category X — Natesto is contraindicated during pregnancy or in women who may become pregnant. Testosterone is teratogenic and may cause fetal harm. Exposure of a fetus to androgens may result in varying degrees of virilization. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
8.3. Nursing Mothers
Although it is not known how much testosterone transfers into human milk, Natesto is contraindicated in nursing women because of the potential for serious adverse reactions in nursing infants.
8.4. Pediatric Use
Safety and efficacy of Natesto has not been established in pediatric patients less than 18 years of age. Improper use may result in acceleration of bone age and premature closure of epiphyses.
8.5. Geriatric Use
There have not been sufficient numbers of geriatric patients involved in controlled clinical studies utilizing Natesto to determine whether efficacy in those over 65 years of age differs from younger subjects.
Of the 306 patients enrolled in the Phase 3 clinical trial utilizing Natesto, 60 were 65 years of age or older, and 9 were 75 years of age or older. There are insufficient long-term safety data in geriatric patients to assess the potential for increased risks of cardiovascular disease and prostate cancer.
Geriatric patients treated with androgens may also be at risk for worsening of signs and symptoms of BPH [see Warnings and Precautions (5.3)].
8.8. Use in Men With Body Mass Index greater than 35 kg/m 2
Safety and efficacy of Natesto in males with body mass index greater than 35 kg/m 2 has not been established.
8.9. Allergic Rhinitis
Serum total testosterone concentrations were decreased by 21 to 24% in males with symptomatic allergic rhinitis, whether treated with nasal decongestants such as oxymetazoline, or left untreated [see Clinical Pharmacology (12.3)].
-
9. DRUG ABUSE AND DEPENDENCE
9.1. Controlled Substance
Natesto contains testosterone, a Schedule III controlled substance in the Controlled Substances Act.
9.2. Abuse
Drug abuse is intentional non-therapeutic use of a drug, even once, for its rewarding psychological and physiological effects. Abuse and misuse of testosterone are seen in male and female adults and adolescents. Testosterone, often in combination with other anabolic androgenic steroids (AAS), and not obtained by prescription through a pharmacy, may be abused by athletes and bodybuilders. There have been reports of misuse by men taking higher doses of legally obtained testosterone than prescribed and continuing testosterone despite adverse events or against medical advice.
Abuse-Related Adverse Reactions
Serious adverse reactions have been reported in individuals who abuse anabolic androgenic steroids and include cardiac arrest, myocardial infarction, hypertrophic cardiomyopathy, congestive heart failure, cerebrovascular accident, hepatotoxicity, and serious psychiatric manifestations, including major depression, mania, paranoia, psychosis, delusions, hallucinations, hostility and aggression.
The following adverse reactions have also been reported in men: transient ischemic attacks, convulsions, hypomania, irritability, dyslipidemias, testicular atrophy, subfertility, and infertility.
The following additional adverse reactions have been reported in women: hirsutism, virilization, deepening of voice, clitoral enlargement, breast atrophy, male-pattern baldness, and menstrual irregularities.
The following adverse reactions have been reported in male and female adolescents: premature closure of bony epiphyses with termination of growth, and precocious puberty.
Because these reactions are reported voluntarily from a population of uncertain size and may include abuse of other agents, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
9.3. Dependence
Behaviors Associated with Addiction
Continued abuse of testosterone and other anabolic steroids, leading to addiction is characterized by the following behaviors:
- Taking greater dosages than prescribed
- Continued drug use despite medical and social problems due to drug use
- Spending significant time to obtain the drug when supplies of the drug are interrupted
- Giving a higher priority to drug use than other obligations
- Having difficulty in discontinuing the drug despite desires and attempts to do so
- Experiencing withdrawal symptoms upon abrupt discontinuation of use
Physical dependence is characterized by withdrawal symptoms after abrupt drug discontinuation or a significant dose reduction of a drug. Individuals taking supratherapeutic doses of testosterone may experience withdrawal symptoms lasting for weeks or months which include depressed mood, major depression, fatigue, craving, restlessness, irritability, anorexia, insomnia, decreased libido and hypogonadotropic hypogonadism.
Drug dependence in individuals using approved doses of testosterone for approved indications has not been documented.
-
10. OVERDOSAGE
No cases of overdose with Natesto have been reported in clinical trials. There is 1 report of acute overdosage by injection of testosterone enanthate: testosterone concentrations of up to 11,400 ng/dL were implicated in a cerebrovascular accident.
Treatment of overdosage would consist of discontinuation of Natesto together with appropriate symptomatic and supportive care.
-
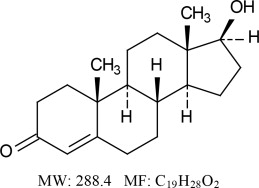
11. DESCRIPTION
Natesto (testosterone) nasal gel is a slightly yellow gel containing 5.5 mg of testosterone in 122.5 mg of Natesto gel for nasal administration. The active pharmacologic ingredient in Natesto is testosterone, an androgen. Testosterone is a white to practically white crystalline powder chemically described as 17β-Hydroxyandrost-4-en-3-one. The structural formula is:
The inactive ingredients are castor oil, oleoyl polyoxylglycerides, and colloidal silicon dioxide.
-
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
Endogenous androgens, including testosterone and dihydrotestosterone (DHT), are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of the prostate, seminal vesicles, penis, and scrotum; the development of male hair distribution, such as facial, pubic, chest, and axillary hair; laryngeal enlargement, vocal cord thickening, alterations in body musculature, and fat distribution. Testosterone and DHT are necessary for the normal development of secondary sex characteristics.
Male hypogonadism, a clinical syndrome resulting from insufficient secretion of testosterone, has two main etiologies. Primary hypogonadism is caused by defects of the gonads, such as Klinefelter's syndrome or Leydig cell aplasia, whereas secondary hypogonadism is the failure of the hypothalamus (or pituitary) to produce sufficient gonadotropins (FSH, LH).
12.3. Pharmacokinetics
Absorption
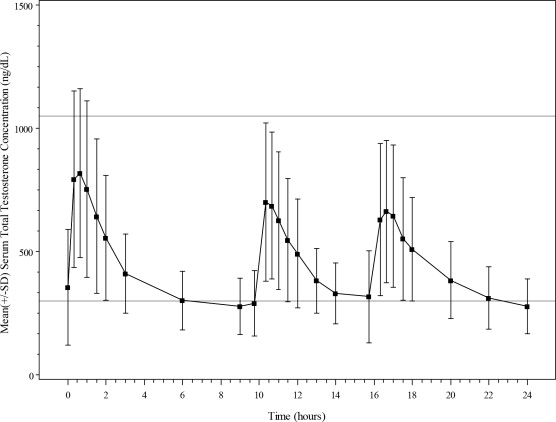
Natesto delivers physiologic amounts of testosterone, producing circulating concentrations that approximate normal testosterone concentrations (i.e., 300 to 1,050 ng/dL) seen in healthy men. The maximum concentration for Natesto is achieved within approximately 40 minutes of administration and has a half-life ranging from 10 to 100 minutes.
Figure 1 summarizes the pharmacokinetic profiles of total testosterone in patients completing 90 days of Natesto treatment administered as 33 mg of testosterone daily (11 mg three times daily).
Figure 1: Mean Serum Total Testosterone Concentrations on Day 90 Following Natesto Administered As 11 mg of Testosterone Three Times Daily (N=69)
The average daily testosterone concentration produced by Natesto administered as 11 mg of testosterone three times daily on Day 90 was 421 (± 116) ng/dL.
Distribution
Circulating testosterone is primarily bound in the serum to sex hormone-binding globulin (SHBG) and albumin. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free), and the rest is loosely bound to albumin and other proteins.
Metabolism
Testosterone is metabolized to various 17-keto steroids through 2 different pathways. The major active metabolites of testosterone are estradiol and dihydrotestosterone (DHT).
DHT concentrations increased in parallel with testosterone concentrations during Natesto treatment. After 90 days of treatment, the mean DHT/testosterone ratio was 0.09 which was within the normal range.
Excretion
About 90% of a dose of testosterone given intramuscularly is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites; about 6% of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver.
Drug Interactions
Use in patients with allergic rhinitis and oxymetazoline: The effects of allergic rhinitis and the use of oxymetazoline on the absorption of testosterone were investigated in a 3-way cross-over clinical study. Eighteen males with seasonal allergic rhinitis received 3 doses of 11 mg of testosterone intranasally (testosterone dose of 33 mg/day) while they were in the asymptomatic, symptomatic, and symptomatic but treated (with oxymetazoline) states using an environmental challenge chamber model.
Serum total testosterone concentrations were decreased by 21 to 24% in males with symptomatic allergic rhinitis. A 2.6% decrease in mean AUC(0-24) and 3.6% decrease in mean C max of total testosterone was observed in males with symptomatic seasonal rhinitis when treated with oxymetazoline 30 minutes prior to Natesto compared to when left untreated. Oxymetazoline does not impact the absorption of testosterone when concomitantly administered with Natesto [see Clinical Pharmacology (12.3)]. Drug interaction potential with nasally administered drugs other than oxymetazoline has not been studied.
-
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenicity
Testosterone has been tested by subcutaneous injection and implantation in mice and rats. In mice, the implant induced cervical-uterine tumors, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats.
-
14. CLINICAL STUDIES
14.1. Testosterone Replacement Therapy
Natesto was evaluated for efficacy in a 90-day, open-label, multicenter study of 306 hypogonadal men. Eligible patients were 18 years of age and older (mean age 54 years) and had morning serum total testosterone concentrations less than 300 ng/dL. Patients were Caucasian (89%), African-American (6%), Asian (5%), or of other ethnicities (less than 1%).
Patients were instructed to self-administer Natesto (11 mg of testosterone) intranasally either two or three times daily.
The primary endpoint was the percentage of patients with an average serum total testosterone concentration (C avg) within the normal range (300 to 1050 ng/dL) on Day 90.
The secondary endpoint was the percentage of patients with a maximum total testosterone concentration (C max) above three predetermined limits: greater than 1500 ng/dL, between 1800 and 2500 ng/dL, and greater than 2500 ng/dL.
A total of 78 hypogonadal men received Natesto (11 mg of testosterone) three times daily (33 mg of testosterone daily). Of these, a total of 73 hypogonadal men were included in the statistical evaluation of efficacy (total testosterone pharmacokinetics) on Day 90 based on the intent-to-treat (ITT) population with last observation carried forward (LOCF). Ninety percent (90%) of these 73 patients had a C avg within the normal range (300 to 1050 ng/dL) on Day 90. The percentages of patients with C avg below the normal range (less than 300 ng/dL) and above the normal range (greater than 1050 ng/dL) on Day 90 were 10% and 0%, respectively.
Table 3 summarizes the mean (SD) serum total testosterone concentrations on Day 90 in 69 patients who had a full pharmacokinetic sampling profile and were treated with Natesto (11 mg of testosterone) three times daily for 90 days.
Table 3: Mean (SD) Serum Total Testosterone Concentrations on Day 90 Following Administration of Natesto (11 mg of testosterone) Three Times Daily C avg = average concentration; C max = maximum concentration; C min = minimum concentration.
Natesto
(11 mg of testosterone)
Three Times Daily
(N=69)C avg (ng/dL) 421 (116) C max (ng/dL) 1044 (378) C min (ng/dL) 215 (74) The percentages of patients with C max greater than 1500 ng/dL and between 1800 and 2500 ng/dL were 15.9% and 1.4%, respectively. No patient had a C max greater than 2500 ng/dL.
-
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. How Supplied
Natesto (testosterone) nasal gel is available as a metered dose pump containing 11 grams of gel dispensed as 60 metered pump actuations. One pump actuation delivers 5.5 mg of testosterone in 0.122 grams of gel.
-
17. PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Use in Men With Known or Suspected Prostate or Breast Cancer
Men with known or suspected prostate or breast cancer should not use Natesto [see Contraindications (4) and Warnings and Precaution (5.3)].
Nasal Adverse Reactions
Nasal adverse reactions, including nasopharyngitis, rhinorrhea, epistaxis, nasal discomfort and nasal scabbing, were reported in clinical trials of Natesto. Advise patients to report any nasal symptoms or signs to their health care professional.
Potential Adverse Reactions With Androgens
Patients should be informed that treatment with androgens may lead to adverse reactions which include:
- Changes in urinary habits such as increased urination at night, trouble starting your urine stream, passing urine many times during the day, having an urge that you have to go to the bathroom right away, having urine accident, being unable to pass urine, and having a weak urine flow.
- Breathing disturbances, including those associated with sleep, or excessive daytime sleepiness.
- Too frequent or persistent erections of the penis.
- Nausea, vomiting, changes in skin color, or ankle swelling.
Patients Should be Advised of the Following Instructions For Use
- Read the Patient Information accompanying each Natesto metered dose pump.
- Prime the pump by depressing it 10 times prior to its first use. No priming is needed with subsequent uses of that pump.
- Administer Natesto intranasally and NOT to other parts of the body. Administer Natesto intranasally three times daily, once in the morning, once in the afternoon and once in the evening (6 to 8 hours apart), preferably at the same time each day.
- Keep Natesto out of the reach of children.
- Report any changes in their state of health, such as changes in urinary habits, breathing, sleep, mood, nasal irritation or rhinitis.
- Never share Natesto with anyone.
-
PATIENT PACKAGE INSERT
Patient Information
Natesto (Na-tes-to) CIII
(testosterone) nasal gelWhat is Natesto?
- Natesto is a prescription medicine that contains testosterone and is used to treat adult males who have low or no testosterone due to certain medical conditions.
- Your healthcare provider will test the testosterone level in your blood before you start and while you are using Natesto.
- It is not known if Natesto is safe or effective to treat men who have low testosterone due to aging.
- It is not known if Natesto is safe or effective in children less than 18 years old. Improper use of Natesto may affect bone growth in children.
- Natesto is a controlled substance (CIII) because it contains testosterone that can be a target for people who abuse prescription medicines. Keep your Natesto in a safe place to protect it. Never give your Natesto to anyone else, even if they have the same symptoms you have. Selling or giving away this medicine may harm others and is against the law.
- Natesto is not meant for use in women.
Who should not use Natesto?
Do not use Natesto if you:
- are a man who has breast cancer
- have or might have prostate cancer
- are pregnant or may become pregnant or are breastfeeding. Natesto may harm your unborn or breastfeeding baby.
Talk to your healthcare provider before using Natesto if you have any of the above conditions. Before using Natesto, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had nose (nasal) or sinus problems or nasal or sinus surgery
- have had a broken nose (fracture) within the past 6 months
- have or have had a fracture of your nose that caused the inside of your nose to be crooked (deviated anterior nasal septum)
- have or have had problems with swelling of the lining of your nose (mucosal inflammatory disorder)
- have breast cancer
- have or might have prostate cancer
- have urinary problems due to an enlarged prostate
- have heart problems
- have liver or kidney problems
- have problems breathing while you sleep (sleep apnea)
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements.
Using Natesto with certain other medicines can affect each other. Especially tell your healthcare provider if you take:- insulin
- medicines that decrease blood clotting
- corticosteroids
How should I use Natesto?
- See the detailed Instructions for Use at the end of this Patient Information for Instructions about the right way to use Natesto.
- Use Natesto exactly as your healthcare provider tells you to. Your healthcare provider will tell you how much Natesto to use and when to use it.
- Natesto is for use in your nose (intranasally) only.
- Natesto can be used with a type of nasal spray called sympathomimetic decongestants such as oxymetazoline. Natesto should not be used with other nasal sprays. It is not known if Natesto is safe and effective when used with other nasal sprays.
What are the possible side effects of Natesto? Natesto may cause serious side effects, including:
- Nose (nasal) problems. Signs and symptoms of nasal problems may include runny nose, congestion, sneezing, nose bleed, nasal discomfort, nasal scabbing, or nasal dryness.
- If you already have enlargement of your prostate gland, your signs and symptoms can get worse while using Natesto. This can include: Increased urination at night, trouble starting your urine stream, having to pass urine many times during the day, having an urge that you have to go to the bathroom right away, having a urine accident, being unable to pass urine or weak urine flow.
- Possible increased risk of prostate cancer. Your healthcare provider should check you for prostate cancer or any other prostate problems before you start and while you use Natesto.
- Changes in red blood cells.
- Blood clots in your legs or lungs. Signs and symptoms of a blood clot in your legs can include leg pain, swelling or redness. Signs and symptoms of a blood clot in your lungs can include difficulty breathing or chest pain.
- Possible increased risk of heart attack or stroke.
- In large doses, Natesto may lower your sperm count.
- Possible increased risk of liver problems. Signs and symptoms of liver problems may include: Nausea or vomiting, yellowing of your skin or whites of your eyes, dark urine, or pain on the right side of your stomach area (abdominal pain).
- Swelling of your ankles, feet, or body, with or without heart failure. This may cause serious problems for people who have heart, kidney, or liver disease.
- Enlarged or painful breasts.
-
Problems breathing while you sleep (sleep apnea).
Call your healthcare provider right away if you have any of the serious side effects listed above. These are not all the possible side effects of Natesto. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
The most common side effects of Natesto include:
- increased prostate specific antigen (a test used to screen for prostate cancer)
- headache
- runny nose
- nose bleeds
- nose pain
- sore throat
- cough
- upper respiratory infection
- sinus infection
- nose scabs
Other side effects include more erections than normal for you or erections that last a long time. How should I store Natesto?
- Store Natesto between 68ºF to 77ºF (20ºC to 25ºC).
- Keep Natesto and all medicines out of the reach of children.
General information about the safe and effective use of Natesto. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Natesto for a condition for which it was not prescribed. Do not give Natesto to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information about Natesto, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Natesto that is written for health professionals. For more information go to www.natesto.com or call 1-833-698-3786.What are the ingredients in Natesto?
Active ingredient: testosterone
Inactive ingredients: castor oil, oleoyl polyoxylglycerides, and colloidal silicon dioxide
Marketed by: Acerus Pharmaceuticals Corporation
Manufactured by: Haupt Pharma Amareg GmbH, Donaustaufer Str. 378, Regensburg, Bavaria D-93055, Germany -
INSTRUCTIONS FOR USE
Instructions for Use
Natesto (Na-tes-to)
CIII (testosterone)
nasal gelSupplies needed to give your Natesto dose:
- 1 Natesto dispenser
- 2 clean, dry tissues
- a clean and flat surface, like a table
- a mirror
Parts of your Natesto dispenser (See Figure A)
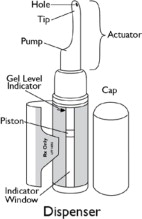
(Figure A) Information about your Natesto:
- Do not blow your nose or sniff for 1 hour after using Natesto.
- Before you use Natesto for the first time, you will need to prime your Natesto pump.
How to prime your Natesto pump:
- Hold your Natesto dispenser over a sink, turn it upside down (invert) and slowly press the pump and release 10 times (See Figure B).
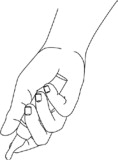
(Figure B) - All the Natesto that comes out when you prime your pump should be rinsed down the sink with warm water.
- If there is any Natesto on the tip of your Natesto actuator after priming, wipe the tip with a clean, dry tissue.
- If any Natesto gel gets on your hands, it is recommended to wash your hands with warm water and soap.
Giving your Natesto dose:
Step 1: Blow your nose.
Step 2: Remove the Natesto cap.
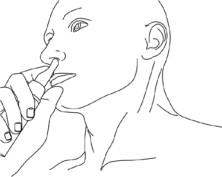
Step 3: While looking in the mirror, put your right first (index) finger on the pump of your Natesto actuator and slowly slide the tip of the actuator up into your left nostril until your finger on the pump touches the bottom of your nose (See Figure C).
(Figure C) Step 4: Gently tilt the Natesto actuator so that the hole in the tip touches the lateral (side) wall of your nostril. This will make sure that Natesto is given in the correct place (See Figure D).
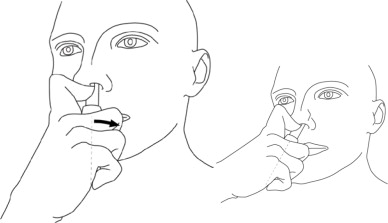
(Figure D) Step 5: With the actuator in place, slowly push the pump down until it stops and remove the actuator from your nose.
Step 6: While looking in the mirror, put your left first (index) finger on the pump of your Natesto actuator and slowly slide the tip of the actuator up into your right nostril until your finger on the pump touches the bottom of your nose (See Figure E).
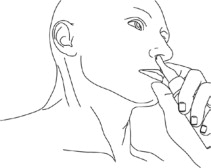
(Figure E) Step 7: Gently tilt the tip of the Natesto actuator so that the hole in the tip touches the lateral (side) wall of your nostril. This will make sure that Natesto is given in the correct place (See Figure F).
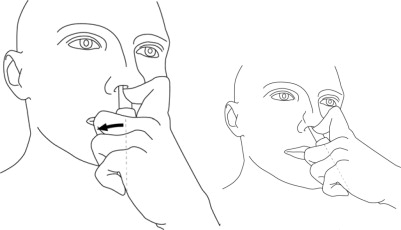
(Figure F) Step 8: Slowly push down on the pump until it stops and remove the actuator from your nose.
Step 9: Wipe the tip of the actuator with a clean, dry tissue.
Step 10: Replace the cap.
Step 11: Press your nostrils together just below the middle of your nose (bridge) and lightly rub them together (See Figure G).
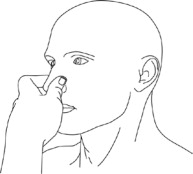
(Figure G) - Replace your Natesto dispenser when the top of the piston inside the dispenser reaches the arrow at the top of the inside label (See Figure H).
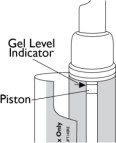
(Figure H) How to throw away your empty Natesto dispenser:
- Safely throw away your empty Natesto dispenser in your household trash.
- Keep Natesto and all medicines out of the reach of children and pets.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Marketed by: Acerus Pharmaceuticals Corporation
Manufactured by: Haupt Pharma Amareg GmbH, Donaustaufer Str. 378, Regensburg, Bavaria D-93055, Germany
Approved: 10/2016
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Natesto Nasal Gel Carton
www.natesto.com
NDC: 42667-5511-1
CIII
Natesto®
(testosterone) nasal gel5.5 mg of testosterone per actuation*
*Each actuation delivers 0.122 g of gel.
Multi-dose pump capable of
dispensing 60 metered pump
actuations.For intranasal use only.
Warning: This package is not
child resistant. Keep out of
reach of children.Total Contents: 11 g / dispenser.
Marketed by:
Acerus Pharmaceuticals Corporation.
Mississauga, ON Canada
Manufactured by:
Haupt Pharma Amareg GmbH
Donaustaufer Sir. 378
Regensburg, Bavaria D93055, Germany
Rx ONLY

-
INGREDIENTS AND APPEARANCE
NATESTO NASAL GEL
testosterone gel, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42667-5511 Route of Administration NASAL DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TESTOSTERONE (UNII: 3XMK78S47O) (TESTOSTERONE - UNII:3XMK78S47O) TESTOSTERONE 5.5 mg in 0.122 g Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) APRICOT SEED OIL (UNII: 54JB35T06A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color white (white actuator, clear body) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42667-5511-1 1 in 1 BOX 06/30/2020 1 11 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205488 05/08/2020 Labeler - Acerus Pharmaceuticals Corporation (243912362) Establishment Name Address ID/FEI Business Operations Acerus Pharmaceuticals Corporation 243912362 label(42667-5511)
Trademark Results [Natesto]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NATESTO 86084664 4761006 Live/Registered |
Trimel BioPharma SRL 2013-10-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.