LORATADINE ODT- loratadine tablet, orally disintegrating
Loratadine ODT by
Drug Labeling and Warnings
Loratadine ODT by is a Otc medication manufactured, distributed, or labeled by CVS Pharmacy, Ranbaxy Pharmaceuticals Inc., Ohm Laboratories Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT (IN EACH TABLET)
- PURPOSE
- USES
-
WARNINGS
Ask a doctor before use if you have
Liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
Do not take more than directed. Taking more than directed may cause drowsiness.
- DIRECTIONS
-
OTHER INFORMATION
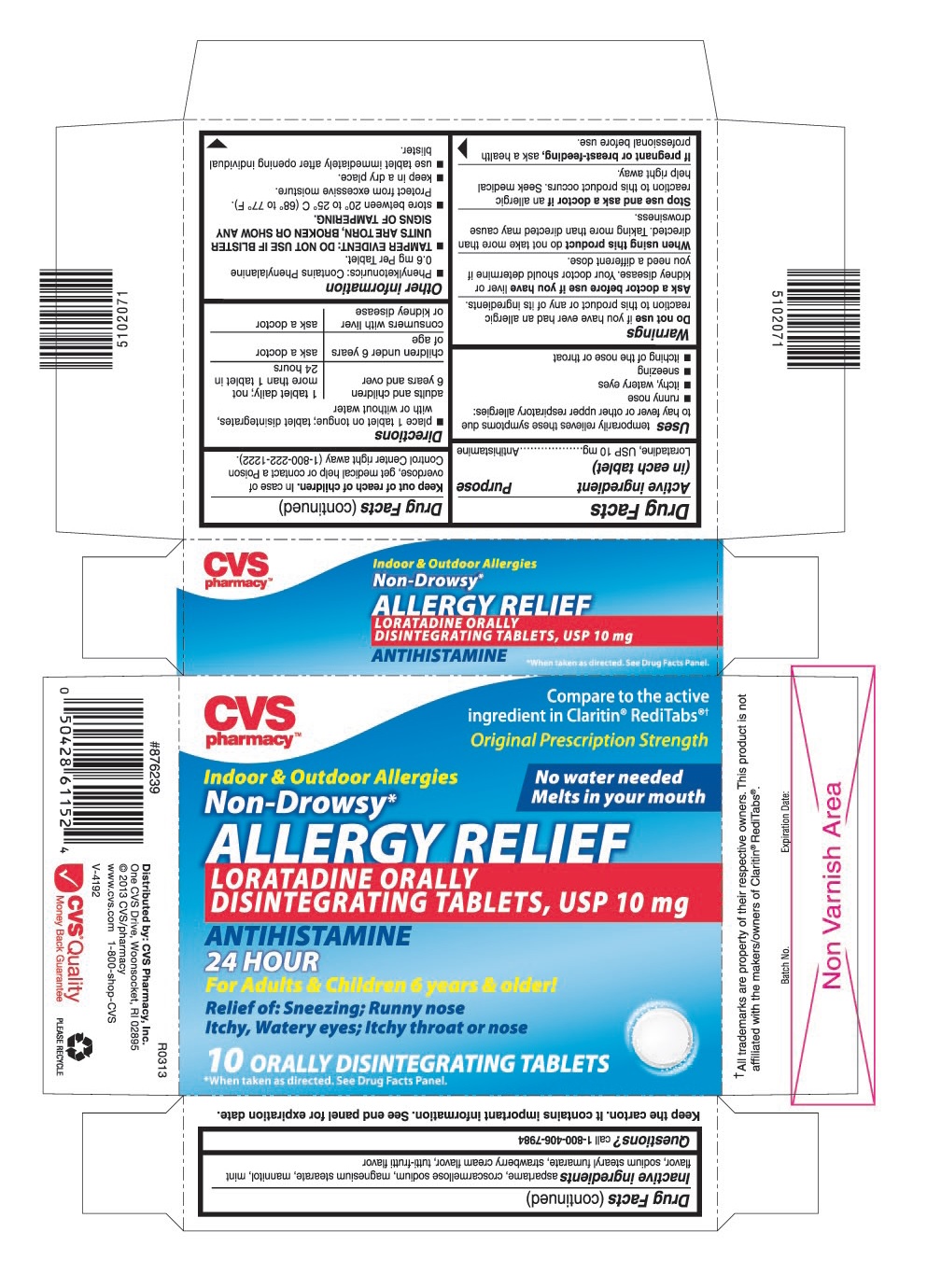
- Phenylketonurics: Contains Phenylalanine 0.6 mg Per Tablet.
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
- store between 20° to 25° C (68° to 77° F). Protect from excessive moisture.
- keep in a dry place.
- use tablet immediately after opening individual blister.
- INACTIVE INGREDIENTS
- QUESTIONS?
-
PRINCIPAL DISPLAY PANEL
Compare to the active ingredient in Claritin®RediTabs®†
Original Prescription Strength
LORATADINE ORALLY DISINTEGRATING TABLETS, 10 mg
For Adults & Children 6 years & older!
Relief of: Sneezing; Runny Nose
Itchy, Watery Eyes; Itchy Throat or Nose
10 ORALLY DISINTEGRATING TABLETS
-
INGREDIENTS AND APPEARANCE
LORATADINE ODT
loratadine tablet, orally disintegratingProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59779-527 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) Product Characteristics Color white (White to Off-White) Score no score Shape ROUND (Flat Faced Beveled Edge) Size 10mm Flavor FRUIT Imprint Code RC17 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59779-527-69 1 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC: 59779-527-31 1 in 1 CARTON 2 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077153 09/27/2007 Labeler - CVS Pharmacy (062312574) Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) Establishment Name Address ID/FEI Business Operations Ohm Laboratories Inc. 051565745 manufacture(59779-527)
Trademark Results [Loratadine ODT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
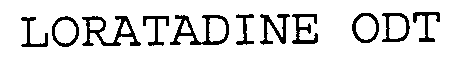 LORATADINE ODT 76601213 not registered Dead/Abandoned |
LEINER HEALTH SERVICES CORP. 2004-07-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.