VELETRI- epoprostenol injection, powder, lyophilized, for solution
Veletri by
Drug Labeling and Warnings
Veletri by is a Prescription medication manufactured, distributed, or labeled by Actelion Pharmaceuticals US, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VELETRI safely and effectively. See full prescribing information for VELETRI.
VELETRI (epoprostenol) for Injection
Initial U.S. Approval: 1995INDICATIONS AND USAGE
VELETRI is a prostanoid vasodilator indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise capacity. Studies establishing effectiveness included predominantly patients with NYHA Functional Class III-IV symptoms and etiologies of idiopathic or heritable PAH or PAH associated with connective tissue diseases. (1)
DOSAGE AND ADMINISTRATION
- Dosage
- - Infusion of VELETRI should be initiated at 2 ng/kg/min and increased in increments of 2 ng/kg/min every 15 minutes or longer until dose-limiting pharmacologic effects are elicited or until a tolerance limit to the drug is established. (2.1)
- - If symptoms of pulmonary hypertension persist or recur after improving - the infusion should be increased by 1- to 2-ng/kg/min increments at intervals sufficient to allow assessment of clinical response; these intervals should be at least 15 minutes. (2.2)
- Administration
- Reconstitution
- - Reconstituted in vial with only 5 mL of either Sterile Water for Injection or Sodium Chloride 0.9% Injection.
- - VELETRI solution reconstituted and immediately diluted to the final concentration in the drug delivery reservoir can be administered per the conditions of use as outlined in Table 1. (2.4 )
- - Solution for chronic delivery should be prepared in a drug delivery reservoir appropriate for the infusion pump. (2.4)
DOSAGE FORMS AND STRENGTHS
10 mL vial with 0.5 mg (500,000 ng) or 1.5 mg (1,500,000 ng) VELETRI. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- VELETRI should be used only by clinicians experienced in the diagnosis and treatment of pulmonary hypertension. (5.1)
- Reconstitute only as directed, with Sterile Water for Injection or Sodium Chloride 0.9% Injection. (5.1)
- Do not abruptly lower the dose or withdraw dosing. All dosing initiation and changes should be closely monitored. (5.3, 5.4)
ADVERSE REACTIONS
- Most common adverse reactions during:
- - Dose Initiation and Escalation: Nausea, vomiting, headache, hypotension, flushing, chest pain, anxiety, dizziness, bradycardia, dyspnea, abdominal pain, musculoskeletal pain, and tachycardia (6.1)
- - Chronic Dosing: Headache, jaw pain, flushing, diarrhea, nausea and vomiting, flu-like symptoms, and anxiety/nervousness (6.1)
To report SUSPECTED ADVERSE REACTIONS, please contact: ACTELION at 1-866-228-3546 or FDA at 1-800-FDA-1088, or http://www.fda.gov/medwatch
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2018
- Dosage
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage
2.2 Dosage Adjustments
2.3 Administration
2.4 Reconstitution
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 General
5.2 Dose Initiation
5.3 Chronic Use and Dose Adjustment
5.4 Withdrawal Effects
5.5 Sepsis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
14 CLINICAL STUDIES
14.1 Clinical Trials in Pulmonary Arterial Hypertension (PAH)
16 HOW SUPPLIED / STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Stability
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
VELETRI is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to improve exercise capacity. Studies establishing effectiveness included predominantly patients with NYHA Functional Class III-IV symptoms and etiologies of idiopathic or heritable PAH or PAH associated with connective tissue diseases.
-
2 DOSAGE AND ADMINISTRATION
Important Note: Reconstitute VELETRI only as directed with Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP. Do not dilute reconstituted solutions of VELETRI or administer it with other parenteral solutions or medications [see Warnings and Precautions (5.1)].
2.1 Dosage
Prepare continuous chronic infusion of VELETRI as directed, and administer through a central venous catheter. Temporary peripheral intravenous infusion may be used until central access is established. Initiate chronic infusion of VELETRI at 2 ng/kg/min and increase in increments of 2 ng/kg/min every 15 minutes or longer until a tolerance limit to the drug is established or further increases in the infusion rate are not clinically warranted. If dose-limiting pharmacologic effects occur, then decrease the infusion rate until VELETRI is tolerated. In clinical trials, the most common dose-limiting adverse events were nausea, vomiting, hypotension, sepsis, headache, abdominal pain, or respiratory disorder (most treatment-limiting adverse events were not serious). If the initial infusion rate of 2 ng/kg/min is not tolerated, use a lower dose.
In the controlled 12-week trial in PAH/SSD, for example, the dose increased from a mean starting dose of 2.2 ng/kg/min. During the first 7 days of treatment, the dose was increased daily to a mean dose of 4.1 ng/kg/min on day 7 of treatment. At the end of week 12, the mean dose was 11.2 ng/kg/min. The mean incremental increase was 2 to 3 ng/kg/min every 3 weeks.
2.2 Dosage Adjustments
Base changes in the chronic infusion rate on persistence, recurrence, or worsening of the patient's symptoms of pulmonary hypertension and the occurrence of adverse events due to excessive doses of VELETRI. In general, expect increases in dose from the initial chronic dose.
Consider increments in dose if symptoms of pulmonary hypertension persist or recur. Adjust the infusion by 1- to 2-ng/kg/min increments at intervals sufficient to allow assessment of clinical response; these intervals should be at least 15 minutes. In clinical trials, incremental increases in dose occurred at intervals of 24 to 48 hours or longer. Following establishment of new chronic infusion rate, observe the patient, and monitor standing and supine blood pressure and heart rate for several hours to ensure that the new dose is tolerated.
During chronic infusion, the occurrence of dose-limiting pharmacological events may necessitate a decrease in infusion rate, but the adverse event may occasionally resolve without dosage adjustment. Make dosage decreases gradually in 2-ng/kg/min decrements every 15 minutes or longer until the dose-limiting effects resolve [see Adverse Reactions (6.1 and 6.2)]. Avoid abrupt withdrawal of VELETRI or sudden large reductions in infusion rates. Except in life-threatening situations (e.g., unconsciousness, collapse, etc.), infusion rates of VELETRI should be adjusted only under the direction of a physician.
In patients receiving lung transplants, doses of epoprostenol were tapered after the initiation of cardiopulmonary bypass.
2.3 Administration
VELETRI, once prepared as directed [see Reconstitution (2.4)], is administered by continuous intravenous infusion via a central venous catheter using an ambulatory infusion pump. During initiation of treatment, VELETRI may be administered peripherally.
Infusion sets with an in-line 0.22 micron filter should be used.
The ambulatory infusion pump used to administer VELETRI should: (1) be small and lightweight, (2) be able to adjust infusion rates in 2-ng/kg/min increments, (3) have occlusion, end-of-infusion, and low-battery alarms, (4) be accurate to ±6% of the programmed rate, and (5) be positive pressure-driven (continuous or pulsatile) with intervals between pulses not exceeding 3 minutes at infusion rates used to deliver VELETRI. The reservoir should be made of polyvinyl chloride, polypropylene, or glass. The infusion pump used in the most recent clinical trials was the CADD-1 HFX 5100 (SIMS Deltec). A 60-inch microbore non-DEHP extension set with proximal antisyphon valve, low priming volume (0.9 mL), and in-line 0.22 micron filter was used during clinical trials.
To avoid potential interruptions in drug delivery, the patient should have access to a backup infusion pump and intravenous infusion sets. Consider a multi-lumen catheter if other intravenous therapies are routinely administered.
2.4 Reconstitution
VELETRI is stable only when reconstituted as directed using Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP. Do not reconstitute or mix VELETRI with any other parenteral medications or solutions prior to or during administration. Each vial is for single use only; discard any unused solution.
Use after reconstitution and immediate dilution to final concentration
Use at room temperature (77°F/25°C)
VELETRI solution reconstituted with 5 mL of Sterile Water for Injection, USP or Sodium Chloride 0.9% Injection, and immediately diluted to the final concentration in the drug delivery reservoir can be administered at room temperature per the conditions of use as outlined in Table 1.
Table 1: Maximum duration of administration (hours) at room temperature (77°F/ 25°C) of fully diluted solutions in the drug delivery reservoir* Final concentration range Immediate administration If stored for up to 8 days at 36° to 46°F (2° to 8°C) - * Short excursions at 104°F (40°C) are permitted for up to:
2 hours for concentrations below 15,000 ng/mL
4 hours for concentrations between 15,000 ng/mL and 60,000 ng/mL
8 hours for concentrations above 60,000 ng/mL0.5mg vial ≥3,000 ng/mL and <15,000 ng/mL 48 hours 24 hours 1.5mg vial ≥15,000 ng/mL and < 60,000 ng/mL 48 hours 48 hours ≥60,000 ng/mL 72 hours 48 hours Use at higher temperatures >77°F up to 104°F (>25° to 40°C)
Temperatures greater than 77°F and up to 86°F (>25°C to 30°C): A single reservoir of fully diluted solution of 60,000 ng/mL or above of VELETRI prepared as directed can be administered (either immediately or after up to 8 days storage at 36° to 46°F (2° to 8°C))for up to 48 hours. For diluted solutions of less than 60,000 ng/mL, pump reservoirs should be changed every 24 hours.
Temperatures up to 104°F (40°C): Fully diluted solutions of 60,000 ng/mL or above of VELETRI, prepared as directed, can be immediately administered for periods up to 24 hours.
Do not expose this solution to direct sunlight.
A concentration for the solution of VELETRI should be selected that is compatible with the infusion pump being used with respect to minimum and maximum flow rates, reservoir capacity, and the infusion pump criteria listed above. VELETRI, when administered chronically, should be prepared in a drug delivery reservoir appropriate for the infusion pump. Outlined in Table 2 are directions for preparing different concentrations of VELETRI. Each vial is for single use only; discard any unused solution.
Table 2: Reconstitution and Dilution Instructions To make 100 mL of solution with Final Concentration (ng/mL) of: Directions: - * Higher concentrations may be prepared for patients who receive VELETRI long-term.
Using the 0.5 mg vial 3,000 ng/ml Dissolve contents of one 0.5 mg vial with 5 mL of Sterile Water for Injection, USP or Sodium Chloride 0.9% Injection, USP. Withdraw 3 mL of the vial contents and add to a sufficient volume of the identical diluent to make a total of 100 mL. 5,000 ng/mL Dissolve contents of one 0.5 mg vial with 5 mL of Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP. Withdraw entire vial contents and add to a sufficient volume of the identical diluent to make a total of 100 mL. 10,000 ng/ml Dissolve contents of two 0.5 mg vials each with 5 mL of Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP.
Withdraw entire vial contents and add to a sufficient volume of the identical diluent to make a total of 100 mL.Using the 1.5 mg vial 15,000 ng/mL* Dissolve contents of one 1.5 mg vial with 5 mL of Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP.
Withdraw entire vial contents and add to a sufficient volume of the identical diluent to make a total of 100 mL.30,000 ng/mL* Dissolve contents of two 1.5 mg vials each with 5 mL of Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP.
Withdraw entire vial contents and add to a sufficient volume of the identical diluent to make a total of 100 mL.Infusion rates may be calculated using the following formula:
Infusion Rate (mL/hr) = [Dose (ng/kg/min) × Weight (kg) × 60 min/hr] Final Concentration (ng/mL) Tables 3 to 7 provide infusion delivery rates for doses up to 16 ng/kg/min based upon patient weight, drug delivery rate, and concentration of the solution of VELETRI to be used. These tables may be used to select the most appropriate concentration of VELETRI that will result in an infusion rate between the minimum and maximum flow rates of the infusion pump and that will allow the desired duration of infusion from a given reservoir volume. For infusion/dose rates lower than those listed in Tables 3 to 7, it is recommended that the pump rate be set by a healthcare professional such that steady state is achieved in the patient, keeping in mind the half life of epoprostenol is no more than six minutes. Higher infusion rates, and therefore, more concentrated solutions may be necessary with long-term administration of VELETRI.
Table 3: Infusion Rates for VELETRI at a Concentration of 3,000 ng/mL Dose or Drug Delivery Rate (ng/kg/min) Patient weight
(kg)2 3 4 5 Infusion Delivery Rate (mL/hr) 20 --- 1.2 1.6 2.0 30 1.2 1.8 2.4 3.0 40 1.6 2.4 3.2 4.0 50 2.0 3.0 4.0 --- 60 2.4 3.6 --- --- 70 2.8 --- --- --- 80 3.2 --- --- --- 90 3.6 --- --- --- 100 4.0 --- --- --- Table 4: Infusion Rates for VELETRI at a Concentration of 5,000 ng/mL Dose or Drug Delivery Rate (ng/kg/min) Patient weight
(kg)2 4 6 8 10 12 14 Infusion Delivery Rate (mL/hr) 20 --- 1.0 1.4 1.9 2.4 2.9 3.4 30 --- 1.4 2.2 2.9 3.6 --- --- 40 1.0 1.9 2.9 3.8 --- --- --- 50 1.2 2.4 3.6 --- --- --- --- 60 1.4 2.9 --- --- --- --- --- 70 1.7 3.4 --- --- --- --- --- 80 1.9 3.8 --- --- --- --- --- 90 2.2 --- --- --- --- --- --- 100 2.4 --- --- --- --- --- --- Table 5: Infusion Rates for VELETRI at a Concentration of 10,000 ng/mL Dose or Drug Delivery Rate (ng/kg/min) Patient weight
(kg)4 6 8 10 12 14 16 Infusion Delivery Rate (mL/hr) 20 --- --- 1.0 1.2 1.4 1.7 1.9 30 --- 1.1 1.4 1.8 2.2 2.5 2.9 40 1.0 1.4 1.9 2.4 2.9 3.4 3.8 50 1.2 1.8 2.4 3.0 3.6 --- --- 60 1.4 2.2 2.9 3.6 --- --- --- 70 1.7 2.5 3.4 --- --- --- --- 80 1.9 2.9 3.8 --- --- --- --- 90 2.2 3.2 --- --- --- --- --- 100 2.4 3.6 --- --- --- --- --- Table 6: Infusion Rates for VELETRI at a Concentration of 15,000 ng/mL Dose or Drug Delivery Rate (ng/kg/min) Patient weight (kg) 4 6 8 10 12 14 16 Infusion Delivery Rate (mL/hr) 20 --- --- --- --- 1.0 1.1 1.3 30 --- --- 1.0 1.2 1.4 1.7 1.9 40 --- 1.0 1.3 1.6 1.9 2.2 2.6 50 --- 1.2 1.6 2.0 2.4 2.8 3.2 60 1.0 1.4 1.9 2.4 2.9 3.4 3.8 70 1.1 1.7 2.2 2.8 3.4 3.9 --- 80 1.3 1.9 2.6 3.2 3.8 --- --- 90 1.4 2.2 2.9 3.6 --- --- --- 100 1.6 2.4 3.2 4.0 --- --- --- Table 7: Infusion Rates for VELETRI at a Concentration of 30,000 ng/mL Dose or Drug Delivery Rate (ng/kg/min) Patient weight (kg) 6 8 10 12 14 16 30 --- --- --- --- --- 1.0 40 --- --- --- 1.0 1.1 1.3 50 --- --- 1.0 1.2 1.4 1.6 60 --- 1.0 1.2 1.4 1.7 1.9 70 --- 1.1 1.4 1.7 2.0 2.2 80 1.0 1.3 1.6 1.9 2.2 2.6 90 1.1 1.4 1.8 2.2 2.5 2.9 100 1.2 1.6 2.0 2.4 2.8 3.2 - * Short excursions at 104°F (40°C) are permitted for up to:
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
A large study evaluating the effect of epoprostenol on survival in NYHA Class III and IV patients with congestive heart failure due to severe left ventricular systolic dysfunction was terminated after an interim analysis of 471 patients revealed a higher mortality in patients receiving epoprostenol plus conventional therapy than in those receiving conventional therapy alone. The chronic use of VELETRI in patients with congestive heart failure due to severe left ventricular systolic dysfunction is therefore contraindicated.
Some patients with pulmonary hypertension have developed pulmonary edema during dose initiation, which may be associated with pulmonary veno-occlusive disease. VELETRI should not be used chronically in patients who develop pulmonary edema during dose initiation.
VELETRI is also contraindicated in patients with known hypersensitivity to the drug or to structurally related compounds.
-
5 WARNINGS AND PRECAUTIONS
5.1 General
Reconstitute VELETRI only as directed using Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP. Do not mix VELETRI with any other parenteral medications or solutions prior to or during administration.
VELETRI should be used only by clinicians experienced in the diagnosis and treatment of pulmonary hypertension. Carefully establish the diagnosis of idiopathic or heritable PAH or PAH-CTD.
5.2 Dose Initiation
VELETRI is a potent pulmonary and systemic vasodilator. Initiate VELETRI in a setting with adequate personnel and equipment for physiologic monitoring and emergency care. Dose initiation has been performed during right heart catheterization and without cardiac catheterization. During dose initiation, asymptomatic increases in pulmonary artery pressure coincident with increases in cardiac output occurred rarely. In such cases, consider dose reduction, but such an increase does not imply that chronic treatment is contraindicated.
5.3 Chronic Use and Dose Adjustment
During chronic use, deliver VELETRI continuously on an ambulatory basis through a permanent indwelling central venous catheter. Unless contraindicated, administer anticoagulant therapy to patients receiving VELETRI to reduce the risk of pulmonary thromboembolism or systemic embolism through a patent foramen ovale. To reduce the risk of infection, use aseptic technique in the reconstitution and administration of VELETRI and in routine catheter care. Because epoprostenol is metabolized rapidly, even brief interruptions in the delivery of VELETRI may result in symptoms associated with rebound pulmonary hypertension including dyspnea, dizziness, and asthenia. Intravenous therapy with VELETRI will likely be needed for prolonged periods, possibly years, so consider the patient's capacity to accept and care for a permanent intravenous catheter and infusion pump.
Based on clinical trials, the acute hemodynamic response (reduction in pulmonary artery resistance) to epoprostenol did not correlate well with improvement in exercise tolerance or survival during chronic use of epoprostenol. Adjust dosage of VELETRI during chronic use at the first sign of recurrence or worsening of symptoms attributable to pulmonary hypertension or the occurrence of adverse events associated with epoprostenol [see Dosage and Administration (2)]. Following dosage adjustments, monitor standing and supine blood pressure and heart rate closely for several hours.
5.4 Withdrawal Effects
Abrupt withdrawal (including interruptions in drug delivery) or sudden large reductions in dosage of VELETRI may result in symptoms associated with rebound pulmonary hypertension, including dyspnea, dizziness, and asthenia. In clinical trials, one Class III primary pulmonary hypertension patient's death was judged attributable to the interruption of epoprostenol. Avoid abrupt withdrawal.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During clinical trials, adverse events were classified as follows: (1) adverse events during dose initiation and escalation, (2) adverse events during chronic dosing, and (3) adverse events associated with the drug delivery system.
Adverse Events during Dose Initiation and Escalation
During early clinical trials, epoprostenol was increased in 2-ng/kg/min increments until the patients developed symptomatic intolerance. The most common adverse events and the adverse events that limited further increases in dose were generally related to vasodilation, the major pharmacologic effect of epoprostenol. The most common dose-limiting adverse events (occurring in ≥1% of patients) were nausea, vomiting, headache, hypotension, and flushing, but also include chest pain, anxiety, dizziness, bradycardia, dyspnea, abdominal pain, musculoskeletal pain, and tachycardia. Table 8 lists the adverse events reported during dose initiation and escalation in decreasing order of frequency.
Table 8: Adverse Events during Dose Initiation and Escalation Adverse Events Occurring
in ≥1% of PatientsEpoprostenol
(n = 391)Flushing 58% Headache 49% Nausea/vomiting 32% Hypotension 16% Anxiety, nervousness, agitation 11% Chest pain 11% Dizziness 8% Bradycardia 5% Abdominal pain 5% Musculoskeletal pain 3% Dyspnea 2% Back pain 2% Sweating 1% Dyspepsia 1% Hypesthesia/paresthesia 1% Tachycardia 1% Adverse Events during Chronic Administration:
Interpretation of adverse events is complicated by the clinical features of PAH, which are similar to some of the pharmacologic effects of epoprostenol (e.g., dizziness, syncope). Adverse events which may be related to the underlying disease include dyspnea, fatigue, chest pain, edema, hypoxia, right ventricular failure, and pallor. Several adverse events, on the other hand, can clearly be attributed to epoprostenol. These include hypotension, bradycardia, tachycardia, pulmonary edema, bleeding at various sites, thrombocytopenia, headache, abdominal pain, pain (unspecified), sweating, rash, arthralgia, jaw pain, flushing, diarrhea, nausea and vomiting, flu-like symptoms, anxiety/nervousness, and agitation. In addition, chest pain, fatigue, and pallor have been reported during epoprostenol therapy, and a role for the drug in these events cannot be excluded.
Adverse Events during Chronic Administration for Idiopathic or Heritable PAH:
In an effort to separate the adverse effects of the drug from the adverse effects of the underlying disease, Table 9 lists adverse events that occurred at a rate at least 10% greater on epoprostenol than on conventional therapy in controlled trials for idiopathic or heritable PAH.
Table 9: Adverse Events Regardless of Attribution Occurring in Patients with Idiopathic or Heritable PAH with ≥ 10% Difference between Epoprostenol and Conventional Therapy Alone Adverse Event Epoprostenol
(n = 52)Conventional Therapy
(n = 54)Occurrence More Common With Epoprostenol General Chills/fever/sepsis/flu-like symptoms 25% 11% Cardiovascular Tachycardia 35% 24% Flushing 42% 2% Gastrointestinal Diarrhea 37% 6% Nausea/vomiting 67% 48% Musculoskeletal Jaw pain 54% 0% Myalgia 44% 31% Nonspecific musculoskeletal pain 35% 15% Neurological Anxiety/nervousness/tremor 21% 9% Dizziness 83% 70% Headache 83% 33% Hypesthesia, hyperesthesia, paresthesia 12% 2% Thrombocytopenia has been reported during uncontrolled clinical trials in patients receiving epoprostenol.
Adverse Events during Chronic Administration for PAH/SSD
In an effort to separate the adverse effects of the drug from the adverse effects of the underlying disease, Table 10 lists adverse events that occurred at a rate at least 10% greater on epoprostenol in the controlled trial.
Table 10: Adverse Events Regardless of Attribution Occurring in Patients with PAH/SSD With ≥10% Difference Between Epoprostenol and Conventional Therapy Alone Adverse Event Epoprostenol
(n = 56)Conventional Therapy
(n = 55)Cardiovascular Flushing 23% 0% Hypotension 13% 0% Gastrointestinal Anorexia 66% 47% Nausea/vomiting 41% 16% Diarrhea 50% 5% Musculoskeletal Jaw pain 75% 0% Pain/neck pain/arthralgia 84% 65% Neurological Headache 46% 5% Skin and Appendages Skin ulcer 39% 24% Eczema/rash/urticaria 25% 4% Although the relationship to epoprostenol administration has not been established, pulmonary embolism has been reported in several patients taking epoprostenol and there have been reports of hepatic failure.
Adverse Events Attributable to the Drug Delivery System
Chronic infusions of epoprostenol are delivered using a small, portable infusion pump through an indwelling central venous catheter. During controlled PAH trials of up to 12 weeks' duration, the local infection rate was about 18%, and the rate for pain was about 11%. During long-term follow-up, sepsis was reported at a rate of 0.3 infections/patient per year in patients treated with epoprostenol. This rate was higher than reported in patients using chronic indwelling central venous catheters to administer parenteral nutrition, but lower than reported in oncology patients using these catheters. Malfunctions in the delivery system resulting in an inadvertent bolus of or a reduction in epoprostenol were associated with symptoms related to excess or insufficient epoprostenol, respectively.
6.2 Post-Marketing Experience
In addition to adverse reactions reported from clinical trials, the following events have been identified during post-approval use of epoprostenol. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to epoprostenol.
Blood and Lymphatic: Anemia, hypersplenism, pancytopenia, splenomegaly.
Cardiac: High output cardiac failure (consider dose reduction) [see Dosage Adjustments (2.2), Dose Initiation (5.2), and Withdrawal Effects (5.4)].
Endocrine and Metabolic: Hyperthyroidism
-
7 DRUG INTERACTIONS
Additional reductions in blood pressure may occur when VELETRI is administered with diuretics, antihypertensive agents, or other vasodilators. When other antiplatelet agents or anticoagulants are used concomitantly, there is the potential for VELETRI to increase the risk of bleeding. However, patients receiving infusions of epoprostenol in clinical trials were maintained on anticoagulants without evidence of increased bleeding. In clinical trials, epoprostenol was used with digoxin, diuretics, anticoagulants, oral vasodilators, and supplemental oxygen.
In a pharmacokinetic substudy in patients with congestive heart failure receiving furosemide or digoxin in whom therapy with epoprostenol was initiated, apparent oral clearance values for furosemide (n = 23) and digoxin (n = 30) were decreased by 13% and 15%, respectively, on the second day of therapy and had returned to baseline values by day 87. The change in furosemide clearance value is not likely to be clinically significant. However, patients on digoxin may show elevations of digoxin concentrations after initiation of therapy with epoprostenol, which may be clinically significant in patients prone to digoxin toxicity.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B. Reproductive studies have been performed in pregnant rats and rabbits at doses up to 100 mcg/kg/day (600 mcg/m2/day in rats, 2.5 times the recommended human dose, and 1,180 mcg/m2/day in rabbits, 4.8 times the recommended human dose based on body surface area) and have revealed no evidence of impaired fertility or harm to the fetus due to epoprostenol. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.2 Labor and Delivery
The use of epoprostenol during labor, vaginal delivery, or cesarean section has not been adequately studied in humans.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when VELETRI is administered to a nursing woman.
8.5 Geriatric Use
Clinical studies of epoprostenol in pulmonary hypertension did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
Signs and symptoms of excessive doses of epoprostenol during clinical trials are the expected dose-limiting pharmacologic effects of epoprostenol, including flushing, headache, hypotension, tachycardia, nausea, vomiting, and diarrhea. Treatment will ordinarily require dose reduction of epoprostenol.
One patient with secondary pulmonary hypertension accidentally received 50 mL of an unspecified concentration of epoprostenol. The patient vomited and became unconscious with an initially unrecordable blood pressure. Epoprostenol was discontinued and the patient regained consciousness within seconds. In clinical practice, fatal occurrences of hypoxemia, hypotension, and respiratory arrest have been reported following overdosage of epoprostenol.
Single intravenous doses of epoprostenol at 10 and 50 mg/kg (2,703 and 27,027 times the recommended acute phase human dose based on body surface area) were lethal to mice and rats, respectively. Symptoms of acute toxicity were hypoactivity, ataxia, loss of righting reflex, deep slow breathing, and hypothermia.
-
11 DESCRIPTION
Epoprostenol sodium is the sodium salt of epoprostenol, formulated as a sterile lyophilized powder for intravenous (IV) administration. Each vial of VELETRI contains epoprostenol sodium equivalent to either 0.5 mg (500,000 ng) or 1.5 mg (1,500,000 ng) epoprostenol, 100 mg sucrose, and 50 mg arginine. Sodium hydroxide is added to adjust pH.
Epoprostenol (PGI2, PGX, prostacyclin), a metabolite of arachidonic acid, is a naturally occurring prostaglandin with potent vasodilatory activity and inhibitory activity of platelet aggregation.
Epoprostenol is (5Z,9a,11a,13E,15S)-6,9-epoxy-11,15-dihydroxyprosta-5,13-dien-1-oic acid. Epoprostenol sodium has a molecular weight of 374.45 and a molecular formula of C20H31NaO5. The structural formula is:
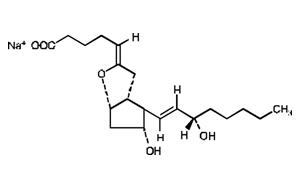
VELETRI is a white to off-white lyophilized powder material. It is reconstituted with Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP.
The reconstituted solution of VELETRI has a pH ranging from 11 to 13 and is increasingly unstable at a lower pH.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Epoprostenol has 2 major pharmacological actions: (1) direct vasodilation of pulmonary and systemic arterial vascular beds, and (2) inhibition of platelet aggregation.
12.2 Pharmacodynamics
In animals, the vasodilatory effects reduce right- and left-ventricular afterload and increase cardiac output and stroke volume. The effect of epoprostenol on heart rate in animals varies with dose. At low doses, there is vagally mediated bradycardia, but at higher doses, epoprostenol causes reflex tachycardia in response to direct vasodilation and hypotension. No major effects on cardiac conduction have been observed. Additional pharmacologic effects of epoprostenol in animals include bronchodilation, inhibition of gastric acid secretion, and decreased gastric emptying.
12.3 Pharmacokinetics
Epoprostenol is rapidly hydrolyzed at neutral pH in blood and is also subject to enzymatic degradation. Animal studies using tritium-labeled epoprostenol have indicated a high clearance (93 mL/kg/min), small volume of distribution (357 mL/kg), and a short half-life (2.7 minutes). During infusions in animals, steady-state plasma concentrations of tritium-labeled epoprostenol were reached within 15 minutes and were proportional to infusion rates.
No available chemical assay is sufficiently sensitive and specific to assess the in vivo human pharmacokinetics of epoprostenol. The in vitro half-life of epoprostenol in human blood at 37°C and pH 7.4 is approximately 6 minutes; therefore, the in vivo half-life of epoprostenol in humans is expected to be no greater than 6 minutes. The in vitro pharmacologic half-life of epoprostenol in human plasma, based on inhibition of platelet aggregation, was similar for males (n = 954) and females (n = 1,024).
Tritium-labeled epoprostenol has been administered to humans in order to identify the metabolic products of epoprostenol. Epoprostenol is metabolized to 2 primary metabolites: 6-keto-PGF1α (formed by spontaneous degradation) and 6,15-diketo-13,14-dihydro-PGF1α (enzymatically formed), both of which have pharmacological activity orders of magnitude less than epoprostenol in animal test systems. The recovery of radioactivity in urine and feces over a 1-week period was 82% and 4% of the administered dose, respectively. Fourteen additional minor metabolites have been isolated from urine, indicating that epoprostenol is extensively metabolized in humans.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. A micronucleus test in rats revealed no evidence of mutagenicity. The Ames test and DNA elution tests were also negative, although the instability of epoprostenol makes the significance of these tests uncertain. Fertility was not impaired in rats given epoprostenol by subcutaneous injection at doses up to 100 mcg/kg/day (600 mcg/m2/day, 2.5 times the recommended human dose [4.6 ng/kg/min or 245.1 mcg/m2/day, IV] based on body surface area).
-
14 CLINICAL STUDIES
14.1 Clinical Trials in Pulmonary Arterial Hypertension (PAH)
Acute Hemodynamic Effects: Acute intravenous infusions of epoprostenol for up to 15 minutes in patients with idiopathic or heritable PAH or PAH associated with scleroderma spectrum of diseases (PAH/SSD) produce dose-related increases in cardiac index (CI) and stroke volume (SV) and dose-related decreases in pulmonary vascular resistance (PVR), total pulmonary resistance (TPR), and mean systemic arterial pressure (SAPm). The effects of epoprostenol on mean pulmonary arterial pressure (PAPm) were variable and minor.
Chronic Infusion in Idiopathic or Heritable PAH:
Hemodynamic Effects: Chronic continuous infusions of epoprostenol in patients with idiopathic or heritable PAH were studied in 2 prospective, open, randomized trials of 8 and 12 weeks' duration comparing epoprostenol plus conventional therapy to conventional therapy alone. Dosage of epoprostenol was determined as described in DOSAGE AND ADMINISTRATION (2) and averaged 9.2 ng/kg/min at study's end. Conventional therapy varied among patients and included some or all of the following: anticoagulants in essentially all patients; oral vasodilators, diuretics, and digoxin in one half to two thirds of patients; and supplemental oxygen in about half the patients. Except for 2 New York Heart Association (NYHA) functional Class II patients, all patients were either functional Class III or Class IV. As results were similar in the 2 studies, the pooled results are described.
Chronic hemodynamic effects were generally similar to acute effects. Increases in CI, SV, and arterial oxygen saturation and decreases in PAPm, mean right atrial pressure (RAPm), TPR, and systemic vascular resistance (SVR) were observed in patients who received epoprostenol chronically compared to those who did not. Table 11 illustrates the treatment-related hemodynamic changes in these patients after 8 or 12 weeks of treatment.
Table 11: Hemodynamics during Chronic Administration of Epoprostenol in Patients with Idiopathic or Heritable PAH
BaselineMean Change from Baseline at End of Treatment Period* Hemodynamic Parameter Epoprostenol
(N = 52)Standard Therapy
(N = 54)Epoprostenol
(N = 48)Standard Therapy
(N = 41)- * At 8 weeks: Epoprostenol N = 10, conventional therapy N = 11
(N is the number of patients with hemodynamic data).
At 12 weeks: Epoprostenol N = 38, conventional therapy N = 30
(N is the number of patients with hemodynamic data).- † Denotes statistically significant difference between Epoprostenol and conventional therapy groups. CI = cardiac index, PAPm = mean pulmonary arterial pressure, PVR = pulmonary vascular resistance, SAPm = mean systemic arterial pressure, SV = stroke volume, TPR = total pulmonary resistance.
CI
(L/min/m2)2.0 2.0 0.3† -0.1 PAPm
(mm Hg)60 60 -5† 1 PVR
(Wood U)16 17 -4† 1 SAPm
(mm Hg)89 91 -4 -3 SV
(mL/beat)44 43 6† -1 TPR
(Wood U)20 21 -5† 1 These hemodynamic improvements appeared to persist when epoprostenol was administered for at least 36 months in an open, nonrandomized study.
Clinical Effects: Statistically significant improvement was observed in exercise capacity, as measured by the 6-minute walk test in patients receiving continuous intravenous epoprostenol plus conventional therapy (N = 52) for 8 or 12 weeks compared to those receiving conventional therapy alone (N = 54). Improvements were apparent as early as the first week of therapy. Increases in exercise capacity were accompanied by statistically significant improvement in dyspnea and fatigue, as measured by the Chronic Heart Failure Questionnaire and the Dyspnea Fatigue Index.
Survival was improved in NYHA functional Class III and Class IV patients with idiopathic or heritable PAH treated with epoprostenol for 12 weeks in a multicenter, open, randomized, parallel study. At the end of the treatment period, 8 of 40 (20%) patients receiving conventional therapy alone died, whereas none of the 41 patients receiving epoprostenol died (p = 0.003).
Chronic Infusion in PAH/Scleroderma Spectrum of Diseases (SSD):
Hemodynamic Effects: Chronic continuous infusions of epoprostenol in patients with PAH/SSD were studied in a prospective, open, randomized trial of 12 weeks' duration comparing epoprostenol plus conventional therapy (N = 56) to conventional therapy alone (N = 55). Except for 5 NYHA functional Class II patients, all patients were either functional Class III or Class IV. Dosage of epoprostenol was determined as described in DOSAGE AND ADMINISTRATION (2) and averaged 11.2 ng/kg/min at study's end. Conventional therapy varied among patients and included some or all of the following: anticoagulants in essentially all patients, supplemental oxygen and diuretics in two thirds of the patients, oral vasodilators in 40% of the patients, and digoxin in a third of the patients. A statistically significant increase in CI, and statistically significant decreases in PAPm, RAPm, PVR, and SAPm after 12 weeks of treatment were observed in patients who received epoprostenol chronically compared to those who did not. Table 12 illustrates the treatment-related hemodynamic changes in these patients after 12 weeks of treatment.
Table 12: Hemodynamics during Chronic Administration of Epoprostenol in Patients with PAH/SSD Baseline Mean Change from Baseline at 12 Weeks Hemodynamic
ParameterEpoprostenol
(N = 56)Conventional
Therapy (N = 55)Epoprostenol
(N = 50)Conventional
Therapy (N = 48)- * Denotes statistically significant difference between Epoprostenol and conventional therapy groups (N is the number of patients with hemodynamic data).
CI = cardiac index, PAPm = mean pulmonary arterial pressure, RAPm = mean right atrial pressure, PVR = pulmonary vascular resistance, SAPm = mean systemic arterial pressure.CI
(L/min/m2)1.9 2.2 0.5* -0.1 PAPm
(mm Hg)51 49 -5* 1 RAPm
(mm Hg)13 11 -1* 1 PVR
(Wood U)14 11 -5* 1 SAPm
(mm Hg)93 89 -8* -1 Clinical Effects: Statistically significant improvement was observed in exercise capacity, as measured by the 6-minute walk, in patients receiving continuous intravenous epoprostenol plus conventional therapy for 12 weeks compared to those receiving conventional therapy alone. Improvements were apparent in some patients at the end of the first week of therapy. Increases in exercise capacity were accompanied by statistically significant improvements in dyspnea and fatigue, as measured by the Borg Dyspnea Index and Dyspnea Fatigue Index. At week 12, NYHA functional class improved in 21 of 51 (41%) patients treated with epoprostenol compared to none of the 48 patients treated with conventional therapy alone. However, more patients in both treatment groups (28/51 [55%] with epoprostenol and 35/48 [73%] with conventional therapy alone) showed no change in functional class, and 2/51 (4%) with epoprostenol and 13/48 (27%) with conventional therapy alone worsened. Of the patients randomized, NYHA functional class data at 12 weeks were not available for 5 patients treated with epoprostenol and 7 patients treated with conventional therapy alone.
No statistical difference in survival over 12 weeks was observed in PAH/SSD patients treated with epoprostenol as compared to those receiving conventional therapy alone. At the end of the treatment period, 4 of 56 (7%) patients receiving epoprostenol died, whereas 5 of 55 (9%) patients receiving conventional therapy alone died.
No controlled clinical trials with epoprostenol have been performed in patients with pulmonary hypertension associated with other diseases.
- * At 8 weeks: Epoprostenol N = 10, conventional therapy N = 11
-
16 HOW SUPPLIED / STORAGE AND HANDLING
16.1 How Supplied
VELETRI is supplied as a sterile lyophilized material in 10 mL vials.
10 mL vial with a white flip-off seal containing epoprostenol sodium equivalent to 0.5 mg (500,000 ng) epoprostenol, is packaged in carton of 1 vial (NDC: 66215-403-01).
10 mL vial with a red flip-off seal containing epoprostenol sodium equivalent to 1.5 mg (1,500,000 ng) epoprostenol, is packaged in carton of 1 vial (NDC: 66215-402-01).
16.2 Storage and Stability
Unopened vials of VELETRI are stable until the date indicated on the package when stored at 68° to 77°F (20° to 25°C). The unopened vial should be kept in the carton and not exposed to direct sunlight.
Use after reconstitution and immediate dilution to final concentration can be found in DOSAGE AND ADMINISTRATION (2.4) Reconstitution, Table 1: Maximum duration of administration (hours) at room temperature (77°F /25°C) of fully diluted solutions in the drug delivery reservoir.
Inspect parenteral drug products for particulate matter and discoloration prior to administration whenever solution and container permit. If either occurs, do not administer.
-
17 PATIENT COUNSELING INFORMATION
Patients receiving VELETRI should receive the following information.
VELETRI must be reconstituted as directed using only Sterile Water for Injection, USP, or Sodium Chloride 0.9% Injection, USP. VELETRI is infused continuously through a permanent indwelling central venous catheter via a small, portable infusion pump. Thus, therapy with VELETRI requires commitment by the patient to drug reconstitution, drug administration, and care of the permanent central venous catheter. Patients must adhere to sterile technique in preparing the drug and in the care of the catheter, and even brief interruptions in the delivery of VELETRI may result in rapid symptomatic deterioration. A patient's decision to receive VELETRI should be based upon the understanding that there is a high likelihood that therapy with VELETRI will be needed for prolonged periods, possibly years. The patient's ability to accept and care for a permanent intravenous catheter and infusion pump should also be carefully considered.
-
PRINCIPAL DISPLAY PANEL - 0.5 mg Vial Carton
NDC: 66215-403-01
Single Dose Vial
Discard Unused PortionVELETRI®
epoprostenol for Injection0.5 mg (500,000 ng)/vial
Sterile, Lyophilized Product
For Intravenous Infusion OnlyRx Only
ACTELION
-
PRINCIPAL DISPLAY PANEL - 1.5 mg Vial Carton
NDC: 66215-402-01
Single Dose Vial
Discard Unused PortionVELETRI®
epoprostenol for Injection1.5 mg (1,500,000 ng)/vial
Sterile, Lyophilized Product
For Intravenous Infusion OnlyRx Only
ACTELION
-
INGREDIENTS AND APPEARANCE
VELETRI
epoprostenol injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66215-403 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength epoprostenol (UNII: DCR9Z582X0) (epoprostenol - UNII:DCR9Z582X0) epoprostenol 0.5 mg in 10 mL Inactive Ingredients Ingredient Name Strength arginine (UNII: 94ZLA3W45F) sucrose (UNII: C151H8M554) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66215-403-01 1 in 1 CARTON 04/22/2010 1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022260 04/22/2010 VELETRI
epoprostenol injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66215-402 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength epoprostenol (UNII: DCR9Z582X0) (epoprostenol - UNII:DCR9Z582X0) epoprostenol 1.5 mg in 10 mL Inactive Ingredients Ingredient Name Strength arginine (UNII: 94ZLA3W45F) sucrose (UNII: C151H8M554) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66215-402-01 1 in 1 CARTON 04/22/2010 1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022260 04/22/2010 Labeler - Actelion Pharmaceuticals US, Inc. (002641228)
Trademark Results [Veletri]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VELETRI 79070519 3818540 Live/Registered |
Actelion Pharmaceuticals Ltd. 2009-06-05 |
 VELETRI 77871287 not registered Dead/Abandoned |
Actelion Pharmaceuticals Ltd. 2009-11-12 |
 VELETRI 76289963 2720582 Live/Registered |
Actelion Pharmaceuticals Ltd. 2001-07-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.