INTRAROSA- prasterone insert
Intrarosa by
Drug Labeling and Warnings
Intrarosa by is a Prescription medication manufactured, distributed, or labeled by AMAG Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use INTRAROSA® safely and effectively. See full prescribing information for INTRAROSA®.
INTRAROSA® (prasterone) vaginal inserts
Initial U.S. Approval: 2016
INDICATIONS AND USAGE
INTRAROSA® is a steroid indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause. (1)
DOSAGE AND ADMINISTRATION
One vaginal insert, once daily at bedtime. (2)
DOSAGE FORMS AND STRENGTHS
Vaginal Insert: 6.5 mg of prasterone. (3)
CONTRAINDICATIONS
Undiagnosed abnormal genital bleeding. (4)
WARNINGS AND PRECAUTIONS
Current or Past History of Breast Cancer. (5.1)
ADVERSE REACTIONS
In four 12-week randomized, placebo-controlled clinical trials, the most common adverse reaction with an incidence ≥ 2 percent was vaginal discharge. (6.1)
In one 52-week open-label clinical trial, the most common adverse reactions with an incidence ≥ 2 percent were vaginal discharge and abnormal Pap smear. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AMAG Pharmaceuticals at 1-877-411-2510 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Current or Past History of Breast Cancer
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In four (4) placebo-controlled, 12-week clinical trials [91% - White Caucasian non-Hispanic women, 7% - Black or African American women, and 2% - "Other" women, average age 58.8 years of age (range 40 to 80 years of age)], vaginal discharge is the most frequently reported treatment-emergent adverse reaction in the INTRAROSA treatment group with an incidence of ≥ 2 percent and greater than reported in the placebo treatment group. There were 38 cases in 665 participating postmenopausal women (5.71 percent) in the INTRAROSA treatment group compared to 17 cases in 464 participating postmenopausal women (3.66 percent) in the placebo treatment group.
In a 52-week non-comparative clinical trial [92% - White Caucasian non-Hispanic women, 6% - Black or African American women, and 2% - "Other" women, average age 57.9 years of age (range 43 to 75 years of age)], vaginal discharge and abnormal Pap smear at 52 weeks were the most frequently reported treatment-emergent adverse reaction in women receiving INTRAROSA with an incidence of ≥ 2 percent. There were 74 cases of vaginal discharge (14.2 percent) and 11 cases of abnormal Pap smear (2.1 percent) in 521 participating postmenopausal women. The eleven (11) cases of abnormal Pap smear at 52 weeks include one (1) case of low-grade squamous intraepithelial lesion (LSIL), and ten (10) cases of atypical cells of undetermined significance (ASCUS).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
INTRAROSA is indicated only in postmenopausal women. There are no data with INTRAROSA use in pregnant women regarding any drug-associated risks. Animal reproduction studies have not been conducted with prasterone.
8.2 Lactation
Risk Summary
INTRAROSA is indicated only in postmenopausal women. There is no information on the presence of prasterone in human milk, the effects on the breastfed infant, or the effects on milk production.
8.5 Geriatric Use
Of the 1522 prasterone-treated postmenopausal women enrolled in the four placebo-controlled 12-week and one open-label 52-week clinical trial, 19 and 11 percent, respectively, were 65 years of age or older.
-
11 DESCRIPTION
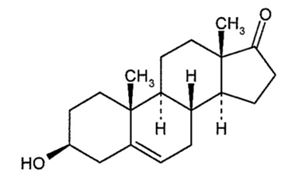
INTRAROSA (prasterone) vaginal insert is a vaginally administered steroid. Prasterone is identified chemically as 3β-hydroxyandrost-5-en-17-one. It has the empirical formula C 19H 28O 2 with a molecular weight of 288.424 g/mol. Prasterone is a white to off-white crystalline powder insoluble in water and soluble in sodium lauryl sulfate (SLS). The structural formula is:
Each INTRAROSA (prasterone) vaginal insert contains 6.5 mg of prasterone in 1.3 ml of off-white hard fat (Witepsol).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Prasterone is an inactive endogenous steroid and is converted into active androgens and/or estrogens. The mechanism of action of INTRAROSA in postmenopausal women with vulvar and vaginal atrophy is not fully established.
12.3 Pharmacokinetics
In a study conducted in postmenopausal women, administration of the INTRAROSA vaginal insert once daily for 7 days resulted in a mean prasterone C max and area under the curve from 0 to 24 hours (AUC 0-24) at Day 7 of 4.4 ng/mL and 56.2 ng·h/mL, respectively, which were significantly higher than those in the group treated with placebo (Table 1). The C max and AUC 0-24 of the metabolites testosterone and estradiol were also slightly higher in women treated with the INTRAROSA vaginal insert compared to those receiving placebo.
Table 1. Cmax and AUC0-24 of Prasterone, Testosterone, and Estradiol on Day 7 Following Daily Administration of Placebo or INTRAROSA (mean ± SD). 1: N=8 Placebo (N=9) INTRAROSA (N=10) Prasterone C max (ng/mL) 1.60 (±0.95) 4.42 (±1.49) AUC 0-24 (ng·h/mL) 24.82 (±14.31) 56.17 (±28.27) Testosterone C max (ng/mL) 0.12 (±0.04) 1 0.15 (±0.05) AUC 0-24 (ng·h/mL) 2.58 (±0.94) 1 2.79 (±0.94) Estradiol C max (pg/mL) 3.33 (±1.31) 5.04 (±2.68) AUC 0-24 (pg·h/mL) 66.49 (±20.70) 96.93 (±52.06) Figure 1. Serum Concentrations of Prasterone (A), Testosterone (B), and Estradiol (C) Measured Over a 24h Period on Day 7 Following Daily Administration of Placebo or INTRAROSA (mean ± SD).
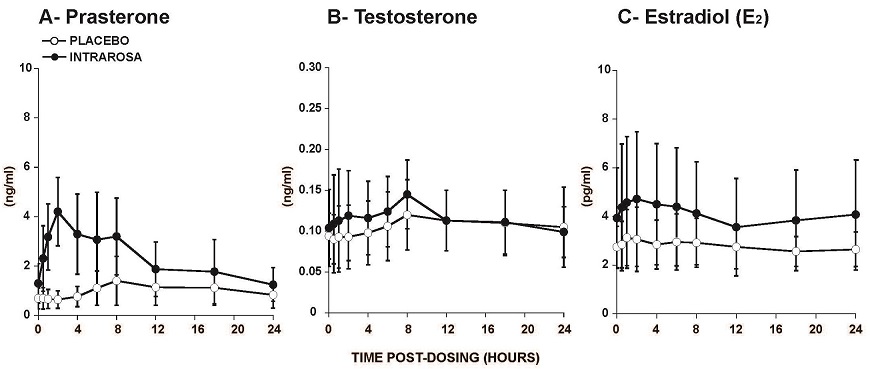
In two primary efficacy trials, daily administration of INTRAROSA vaginal insert for 12 weeks increased mean serum C trough of prasterone and its metabolites testosterone and estradiol by 47%, 21% and 19% from baseline, respectively. This comparison based on C trough may underestimate the magnitude of increase in prasterone and metabolites’ exposure because it does not take into account the overall concentration-time profile following administration of INTRAROSA.
Metabolism
Exogenous prasterone is metabolized in the same manner as endogenous prasterone. Human steroidogenic enzymes such as hydroxysteroid dehydrogenases, 5α-reductases and aromatases transform prasterone into androgens and estrogens.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to evaluate carcinogenic potential have not been conducted with prasterone. Two metabolites of prasterone, testosterone and estradiol, are carcinogenic in animals.
Mutagenesis
Prasterone was not genotoxic in the in vitro bacterial mutagenesis assay (Ames test), the in vitro chromosomal aberrations assay with human peripheral blood lymphocytes, and in vivo in the mouse bone marrow micronucleus assay.
Fertility
Fertility studies were not conducted with prasterone.
-
14 CLINICAL STUDIES
The effectiveness of INTRAROSA on moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause was examined in two primary 12-week placebo-controlled efficacy trials.
The first clinical trial (Trial 1) was a 12-week randomized, double-blind and placebo-controlled trial that enrolled 255 generally healthy postmenopausal women between 40 to 75 years of age (mean 58.6 years) who, at baseline, identified moderate to severe dyspareunia as their most bothersome symptom of vulvar and vaginal atrophy. In addition to moderate to severe dyspareunia, women had ≤ 5% superficial cells on vaginal smear and a vaginal pH > 5. Women were randomized in a 1:1:1 ratio between three treatment groups who received daily INTRAROSA (n=87), one active comparator vaginal insert (n=87), or placebo (n=81). All women were assessed for improvement from Baseline to Week 12 for four co-primary efficacy endpoints: most bothersome moderate to severe symptom of dyspareunia, the percentage of vaginal superficial cells, the percentage of parabasal cells, and vaginal pH.
The second clinical trial (Trial 2) was a 12-week randomized, double-blind and placebo-controlled trial that enrolled 558 generally healthy postmenopausal women between 40 to 80 years of age (mean 59.5 years) who, at baseline, had identified moderate to severe dyspareunia as their most bothersome symptom of vulvar and vaginal atrophy. In addition to dyspareunia, women had ≤ 5% superficial cells on vaginal smear and a vaginal pH > 5. Women were randomized in a 2:1 ratio to receive once daily vaginal insert containing 6.5 mg INTRAROSA (n=376) or placebo (n=182). The primary endpoints and study conduct were the same or similar to those in Trial 1.
The primary efficacy results obtained in the Intent-to-Treat (ITT) population in Trial 1 are shown in Table 2.
Table 2: Efficacy Summary in Primary 12-Week Trial 1: ITT Population (LOCF)
1Difference from placebo =INTRAROSA (Week 12 mean – Baseline mean) – Placebo (Week 12 mean – Baseline mean).
2ANCOVA: Treatment as the main factor and Baseline value as the covariate.Placebo
N = 77
INTRAROSA
N = 81
Dyspareunia
Baseline Mean Severity
Week 12 Mean Severity
Mean Change in Severity (SD)
Difference from Placebo 1
p-value 2
2.58
1.71
-0.87 (0.95)
-
-
2.63
1.36
-1.27 (0.99)
-0.40
0.0132
% Superficial Cells
Baseline Mean
Week 12 Mean
Mean Change (SD)
Difference from Placebo 1
p-value 2
0.73
1.64
0.91 (2.69)
-
-
0.68
6.30
5.62 (5.49)
4.71
<0.0001
% Parabasal Cells
Baseline Mean
Week 12 Mean
Mean Change (SD)
Difference from Placebo 1
p-value 2
68.48
66.86
-1.62 (28.22)
-
-
65.05
17.65
-47.40 (42.50)
-45.77
<0.0001
Vaginal pH
Baseline Mean
Week 12 Mean
Mean Change (SD)
Difference from Placebo 1
p-value 2
6.51
6.31
-0.21 (0.69)
-
-
6.47
5.43
-1.04 (1.00)
-0.83
<0.0001
The primary efficacy results obtained in the Intent-to-Treat (ITT) population in Trial 2 are shown in Table 3.
Table 3: Efficacy Summary in Primary 12-Week Trial 2: ITT Population (LOCF)
1Difference from placebo =INTRAROSA (Week 12 mean – Baseline mean) – Placebo (Week 12 mean – Baseline mean).
2ANCOVA: Treatment as the main factor and Baseline value as the covariate.Placebo
N = 157
INTRAROSA
N = 325
Dyspareunia
Baseline Mean Severity
Week 12 Mean Severity
Mean Change in Severity (SD)
Difference from Placebo 1
p-value 2
2.56
1.50
-1.06 (1.02)
-
-
2.54
1.13
-1.42 (1.00)
-0.35
0.0002
% Superficial Cells
Baseline Mean
Week 12 Mean
Mean Change (SD)
Difference from Placebo 1
p-value 2
1.04
2.78
1.75 (3.33)
-
-
1.02
11.22
10.20 (10.35)
8.46
<0.0001
% Parabasal Cells
Baseline Mean
Week 12 Mean
Mean Change (SD)
Difference from Placebo 1
p-value 2
51.66
39.68
-11.98 (29.58)
-
-
54.25
12.74
-41.51 (36.26)
-29.53
<0.0001
Vaginal pH
Baseline Mean
Week 12 Mean
Mean Change (SD)
Difference from Placebo 1
p-value 2
6.32
6.05
-0.27 (0.74)
-
-
6.34
5.39
-0.94 (0.94)
-0.67
<0.0001
-
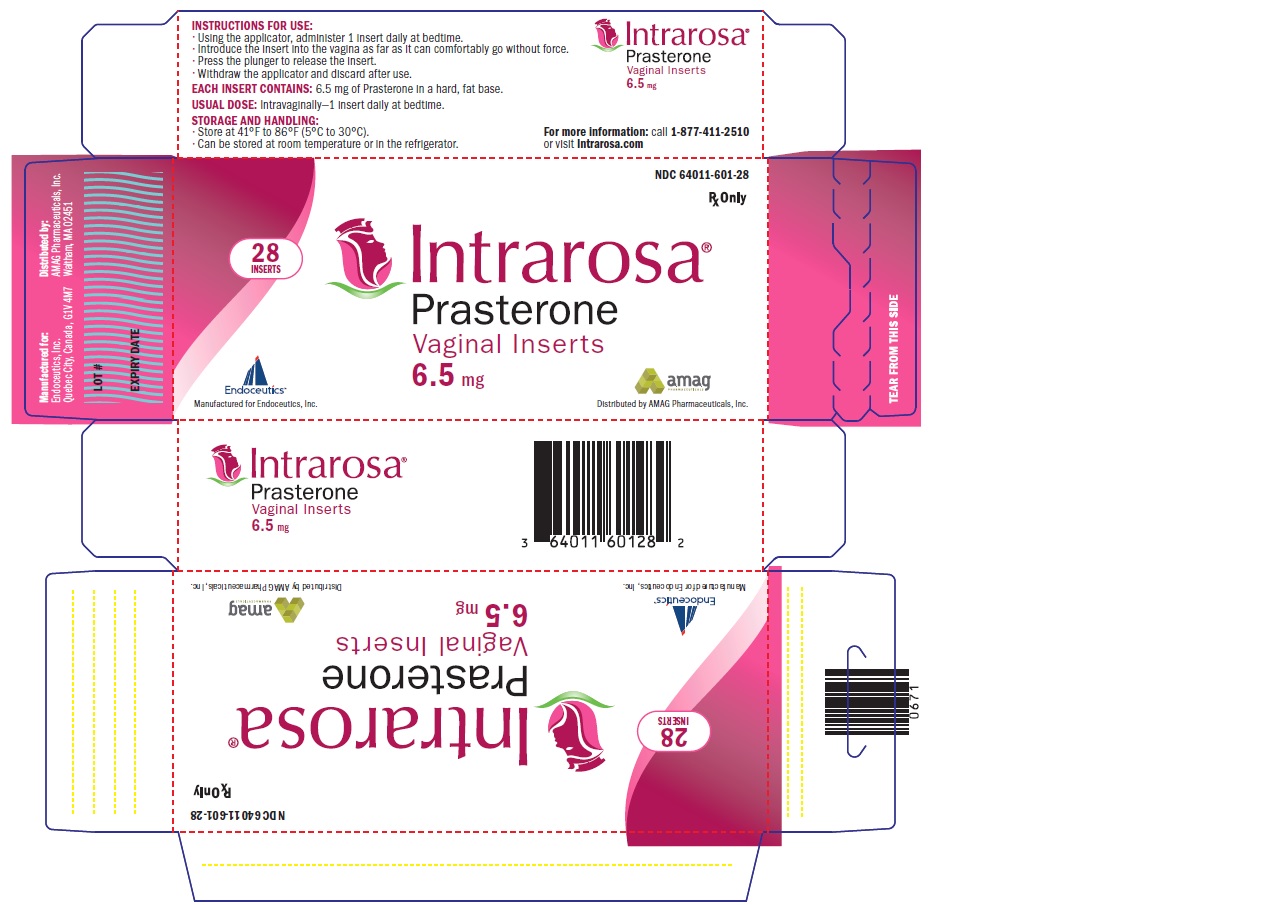
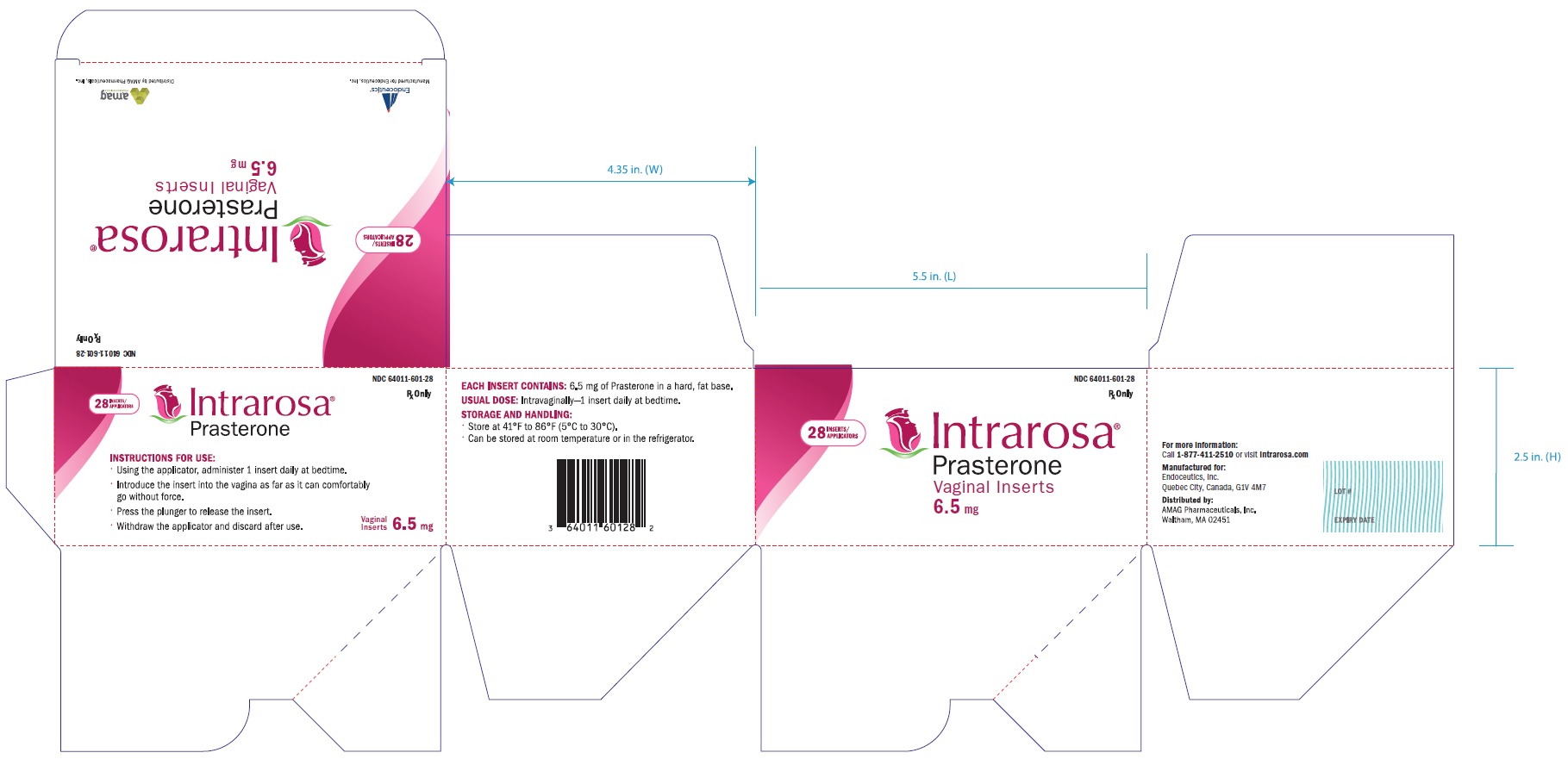
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
INTRAROSA is supplied as white to off-white 1.3 mL solid fat bullet-shaped, smooth vaginal inserts (containing 6.5 mg of prasterone). INTRAROSA is available in small boxes of 4 blister packs containing 7 vaginal inserts (28 vaginal inserts per box). The small box (containing the vaginal inserts) is supplied inside a larger box containing 28 applicators (NDC: 64011-601-28).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read FDA-approved patient labeling (Patient Information and Instructions for Use).
Vaginal Discharge
Inform postmenopausal women that vaginal discharge may occur with INTRAROSA [see Adverse Reactions (6.1)] .
Abnormal Pap Smear Findings
Inform postmenopausal women that abnormal Pap smear findings may occur with INTRAROSA [see Adverse Reactions (6.1)] .
Manufactured for:
Endoceutics, Inc.
Quebec City, Canada, G1V 4M7
Distributed by:
AMAG Pharmaceuticals, Inc.
Waltham, MA 02451
-
PATIENT PACKAGE INSERT
Patient Information
INTRAROSA® (in trah ROE sah)
(prasterone) vaginal inserts
What is INTRAROSA vaginal inserts?
INTRAROSA vaginal inserts are a prescription medicine used in women after menopause to treat moderate to severe pain during sexual intercourse caused by changes in and around the vagina that happen with menopause.
It is not known if INTRAROSA vaginal inserts are safe and effective in children.
Do not use INTRAROSA vaginal inserts if you have vaginal bleeding that has not been checked by your healthcare provider.
Before using INTRAROSA vaginal inserts, tell your healthcare provider about all of your medical conditions, including if you:
- have, have had, or think you may have had breast cancer. Prasterone, an ingredient in INTRAROSA vaginal inserts, is changed in your body to estrogen. Estrogen medicines are not for use in women who have, have had, or think they may have had breast cancer.
- are pregnant or plan to become pregnant. INTRAROSA is only for use in women who are past menopause. It is not known if INTRAROSA vaginal inserts will harm your unborn baby.
- are breastfeeding or plan to breastfeed. INTRAROSA vaginal inserts are only for use in women who are past menopause. It is not known if INTRAROSA passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use INTRAROSA vaginal inserts?
- See the Instructions for Use at the end of this Patient Information for detailed instructions about the right way to use INTRAROSA vaginal inserts.
- Use INTRAROSA vaginal inserts exactly how your healthcare provider tells you to use it.
- Place 1 INTRAROSA vaginal insert in your vagina one time each day at bedtime, using the applicator that comes with INTRAROSA vaginal inserts.
What are the possible side effects of INTRAROSA vaginal inserts?
The most common side effects of INTRAROSA vaginal inserts are vaginal discharge and changes on Pap smear.
These are not all of the possible side effects of INTRAROSA vaginal inserts.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store INTRAROSA vaginal inserts?
- Store INTRAROSA vaginal inserts between 41°F to 86°F (5°C to 30°C).
- INTRAROSA vaginal inserts can be stored at room temperature or in the refrigerator.
Keep INTRAROSA vaginal inserts and all medicines out of the reach of children.
General Information about the safe and effective use of INTRAROSA vaginal inserts.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use INTRAROSA vaginal inserts for a condition for which it was not prescribed. Do not give INTRAROSA vaginal inserts to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about INTRAROSA vaginal inserts that is written for health professionals.
What are the ingredients in INTRAROSA vaginal inserts?
Active ingredient: prasterone
Inactive ingredient: off-white hard fat (Witepsol)
For more information, go to www.intrarosa.com or call 1-877-411-2510.
-
INSTRUCTIONS FOR USE
Instructions for Use
INTRAROSA (in trah ROE sah)
(prasterone) vaginal insertsHow should I use INTRAROSA vaginal inserts?
- INTRAROSA is a vaginal insert that you place in your vagina with an applicator that comes with INTRAROSA vaginal inserts.
- Use 1 INTRAROSA vaginal insert, one time each day at bedtime.
- Each applicator is for one time use only.
- Empty your bladder and wash your hands before handling the vaginal insert and applicator.
- Tear off 1 vaginal insert along the perforations from the 7-vaginal insert strip.
STEP 1
1a. Remove 1 applicator from the package.
1b. Pull back on the plunger until it stops to activate the applicator. The applicator must be activated before use. Place the applicator on a clean surface.
STEP 2
Slowly pull the plastic tabs on the vaginal insert away from each other while keeping the vaginal insert still between your fingers. Carefully remove the vaginal insert from the plastic wrap. If a vaginal insert falls on an unsanitary surface, replace it with a new one.
STEP 3
Place the flat end of the vaginal insert into the open end of the activated applicator as shown. You are now ready to insert the vaginal insert into your vagina.
STEP 4
Hold the applicator between your thumb and middle finger. Leave your index (pointer) finger free to press the applicator plunger after the applicator is inserted into your vagina.
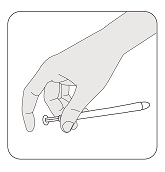
STEP 5
Select the position for insertion of the vaginal insert that is most comfortable for you.
5a. Lying position
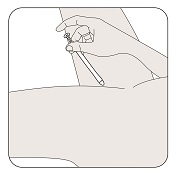
5b. Standing position
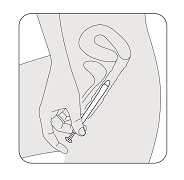
STEP 6
Gently slide the vaginal insert end of the applicator into your vagina as far as it will comfortably go.
Do not use force.
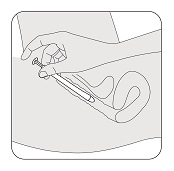
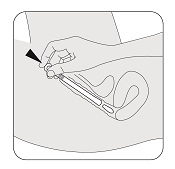
STEP 7
Press the applicator plunger with your index (pointer) finger to release the vaginal insert. Remove the applicator and throw it away after use.
For more information, go to www.intrarosa.com or call AMAG Pharmaceuticals, Inc. at 1-877-411-2510.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
INTRAROSA® is a registered trademark of Endoceutics, Inc.
Manufactured for: Endoceutics, Inc., Quebec City, Canada, G1V 4M7
Distributed by: AMAG Pharmaceuticals, Inc., Waltham, MA 02451
©2018 AMAG Pharmaceuticals, Inc.Issued: February 2018
- PRINCIPAL DISPLAY PANEL - NDC: 64011-601-28 - Small Box (Inserts)
- PRINCIPAL DISPLAY PANEL - NDC: 64011-601-28 - Outmost Large Box (Inserts plus Applicators)
- PRINCIPAL DISPLAY PANEL - NDC: 64011-601-14 - Professional Sample Small Box (Inserts)
- PRINCIPAL DISPLAY PANEL - NDC: 64011-601-14 - Professional Sample Outmost Large Box (Inserts plus Applicators)
-
INGREDIENTS AND APPEARANCE
INTRAROSA
prasterone insertProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 64011-601 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRASTERONE (UNII: 459AG36T1B) (PRASTERONE - UNII:459AG36T1B) PRASTERONE 6.5 mg Inactive Ingredients Ingredient Name Strength HYDROGENATED COCO-GLYCERIDES (UNII: XDD37N2GPR) Product Characteristics Color white (White to off-white) Score no score Shape BULLET Size 28mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64011-601-28 1 in 1 BOX 06/06/2017 1 28 in 1 BOX 1 1 in 1 BLISTER PACK; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug 2 NDC: 64011-601-14 1 in 1 BOX 06/06/2017 2 14 in 1 BOX 2 1 in 1 BLISTER PACK; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208470 06/06/2017 Labeler - AMAG Pharmaceuticals, Inc. (017511155)
Trademark Results [Intrarosa]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 INTRAROSA 86763598 5311971 Live/Registered |
Endoceutics, Inc. 2015-09-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.