FIDAXOMICIN tablet, film coated
Fidaxomicin by
Drug Labeling and Warnings
Fidaxomicin by is a Prescription medication manufactured, distributed, or labeled by Teva Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FIDAXOMICIN TABLETS safely and effectively. See full prescribing information for FIDAXOMICIN TABLETS.
FIDAXOMICIN tablets, for oral use
Initial U.S. Approval: 2011
INDICATIONS AND USAGE
Fidaxomicin tablets are a macrolide antibacterial indicated in adult patients for the treatment of C. difficile-associated diarrhea. (1.1)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of fidaxomicin and other antibacterial drugs, fidaxomicin should be used only to treat infections that are proven or strongly suspected to be caused by C. difficile. (1.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Film-coated tablets: 200 mg (3)
CONTRAINDICATIONS
Fidaxomicin is contraindicated in patients who have known hypersensitivity to fidaxomicin or any other ingredient in fidaxomicin tablets. (4)
WARNINGS AND PRECAUTIONS
- Acute hypersensitivity reactions (angioedema, dyspnea, pruritus, and rash) have been reported. If a severe hypersensitivity reaction occurs, discontinue fidaxomicin. (5.1)
- Fidaxomicin is not expected to be effective for the treatment of other types of infections due to minimal systemic absorption of fidaxomicin.
Fidaxomicin should only be used for the treatment of C. difficile-associated diarrhea. (5.2) - Development of drug-resistant bacteria: Only use fidaxomicin for infection proven or strongly suspected to be caused by C. difficile. (5.3)
ADVERSE REACTIONS
The most common adverse reactions in adults (incidence ≥2%) are nausea, vomiting, abdominal pain, gastrointestinal hemorrhage, anemia, and neutropenia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Teva at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pediatrics: The safety and effectiveness of fidaxomicin have not been established in pediatric patients younger than 6 months of age. (8.4)
Pediatric use information is approved for Cubist Pharmaceuticals LLC's DIFICID® (fidaxomicin) tablets. However, due to Cubist Pharmaceuticals LLC's marketing exclusivity rights, this drug product is not labeled with that information.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Clostridioides difficile-Associated Diarrhea
1.2 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Adult Patients
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Not for Use in Infections Other than C. difficile-Associated Diarrhea
5.3 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Cyclosporine
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Clinical Studies of Fidaxomicin in Adult Patients with CDAD
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Clostridioides difficile-Associated Diarrhea
Fidaxomicin tablets are indicated in adult patients for the treatment of C. difficile-associated diarrhea (CDAD).
1.2 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of fidaxomicin tablets and other antibacterial drugs, fidaxomicin tablets should be used only to treat infections that are proven or strongly suspected to be caused by C. difficile. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Pediatric use information is approved for Cubist Pharmaceuticals LLC's DIFICID® (fidaxomicin) tablets. However, due to Cubist Pharmaceuticals LLC's marketing exclusivity rights, this drug product is not labeled with that information.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Fidaxomicin tablets are available for oral administration as 200 mg tablets. Fidaxomicin tablets are administered orally with or without food.
2.2 Adult Patients
The recommended dosage for adults is one 200 mg fidaxomicin tablet orally twice daily for 10 days.
Pediatric use information is approved for Cubist Pharmaceuticals LLC's DIFICID® (fidaxomicin) tablets. However, due to Cubist Pharmaceuticals LLC's marketing exclusivity rights, this drug product is not labeled with that information.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Fidaxomicin is contraindicated in patients who have known hypersensitivity to fidaxomicin or any other ingredient in fidaxomicin tablets [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Acute hypersensitivity reactions, including dyspnea, rash, pruritus, and angioedema of the mouth, throat, and face have been reported with fidaxomicin. If a severe hypersensitivity reaction occurs, fidaxomicin should be discontinued and appropriate therapy should be instituted.
Some patients with hypersensitivity reactions to fidaxomicin also reported a history of allergy to other macrolides. Physicians prescribing fidaxomicin to patients with a known macrolide allergy should be aware of the possibility of hypersensitivity reactions.
5.2 Not for Use in Infections Other than C. difficile-Associated Diarrhea
Fidaxomicin is not expected to be effective for the treatment of other types of infections due to minimal systemic absorption of fidaxomicin [see Clinical Pharmacology (12.3)]. Fidaxomicin has not been studied for the treatment of infections other than CDAD. Fidaxomicin should only be used for the treatment of CDAD.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
The safety of fidaxomicin 200 mg tablets taken twice a day for 10 days was evaluated in 564 adult patients with CDAD in two active-controlled trials with 86.7% of patients receiving a full course of treatment.
Thirty-three adult patients receiving fidaxomicin (5.9%) withdrew from trials as a result of adverse reactions (AR). The types of AR resulting in withdrawal from the study varied considerably. Vomiting was the primary adverse reaction leading to discontinuation of dosing; this occurred at an incidence of 0.5% in both the fidaxomicin and vancomycin patients in Phase 3 trials. The most common selected adverse reactions occurring in ≥2% of adult patients treated with fidaxomicin are listed in Table 2.
Table 2: Selected Adverse Reactions with an Incidence of ≥2% Reported in Fidaxomicin-Treated Adult Patients in Controlled Trials System Organ Class
Adverse Reaction
Fidaxomicin
(N=564)
Vancomycin
(N=583)
n (%)
n (%)
Blood and Lymphatic System Disorders
Anemia
14 (2%)
12 (2%)
Neutropenia
14 (2%)
6 (1%)
Gastrointestinal Disorders
Nausea
62 (11%)
66 (11%)
Vomiting
41 (7%)
37 (6%)
Abdominal Pain
33 (6%)
23 (4%)
Gastrointestinal Hemorrhage
20 (4%)
12 (2%)
The following adverse reactions were reported in <2% of adult patients taking fidaxomicin tablets in controlled trials:
Gastrointestinal Disorders: abdominal distension, abdominal tenderness, dyspepsia, dysphagia, flatulence, intestinal obstruction, megacolon
Investigations: increased blood alkaline phosphatase, decreased blood bicarbonate, increased hepatic enzymes, decreased platelet count
Metabolism and Nutrition Disorders: hyperglycemia, metabolic acidosis
Skin and Subcutaneous Tissue Disorders: drug eruption, pruritus, rash
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of fidaxomicin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity reactions (dyspnea, angioedema, rash, pruritus)
Pediatric use information is approved for Cubist Pharmaceuticals LLC's DIFICID® (fidaxomicin) tablets. However, due to Cubist Pharmaceuticals LLC's marketing exclusivity rights, this drug product is not labeled with that information.
-
7 DRUG INTERACTIONS
Fidaxomicin and its main metabolite, OP-1118, are substrates of the efflux transporter, P-glycoprotein (P-gp), which is expressed in the gastrointestinal tract.
7.1 Cyclosporine
Cyclosporine is an inhibitor of multiple transporters, including P-gp. When cyclosporine was coadministered with fidaxomicin, plasma concentrations of fidaxomicin and OP-1118 were significantly increased but remained in the ng/mL range [see Clinical Pharmacology (12.3)]. Concentrations of fidaxomicin and OP-1118 may also be decreased at the site of action (i.e., gastrointestinal tract) via P-gp inhibition; however, concomitant P-gp inhibitor use had no attributable effect on safety or treatment outcome of fidaxomicin-treated adult patients in controlled clinical trials. Based on these results, fidaxomicin may be coadministered with P-gp inhibitors and no dose adjustment is recommended.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data on use of fidaxomicin in pregnant women are insufficient to inform any drug-associated risk for major birth defects, miscarriage or adverse maternal or fetal outcomes. Embryo-fetal reproduction studies in rats and rabbits dosed intravenously during organogenesis revealed no evidence of harm to the fetus at fidaxomicin and OP-1118 (its main metabolite) exposures 65-fold or higher than the clinical exposure at the fidaxomicin recommended dose [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In pregnant rats, fidaxomicin was administered intravenously at doses of 4, 8, and 15 mg/kg/day from gestation day 6 through 17 (during the period of organogenesis). No embryo/fetal effects were noted in this study at exposures (AUC) 193-fold higher for fidaxomicin, and 65-fold higher for OP-1118 than the clinical exposure at the fidaxomicin recommended dose.
In pregnant rabbits, fidaxomicin was administered intravenously at doses of 2, 4, and 7.5 mg/kg/day from gestation day 6 through 18 (during the period of organogenesis). No embryo/fetal effects were noted in this study at exposures 66-fold higher for fidaxomicin, and 245-fold higher for OP-1118 than the clinical exposure at the fidaxomicin recommended dose.
8.2 Lactation
Risk Summary
There is no information on the presence of fidaxomicin or its main metabolite, OP-1118, in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for fidaxomicin and any potential adverse effects on the breastfed infant from fidaxomicin or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of fidaxomicin have not been established in pediatric patients younger than 6 months of age.
Pediatric use information is approved for Cubist Pharmaceuticals LLC's DIFICID® (fidaxomicin) tablets. However, due to Cubist Pharmaceuticals LLC's marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
Of the total number of patients in controlled trials of fidaxomicin, 50% were 65 years of age and over, while 31% were 75 and over. No overall differences in safety or effectiveness of fidaxomicin compared to vancomycin were observed between these subjects and younger subjects.
In controlled trials, elderly patients (≥65 years of age) had higher plasma concentrations of fidaxomicin and its main metabolite, OP-1118, versus non-elderly patients (<65 years of age) [see Clinical Pharmacology (12.3)]. However, greater exposures in elderly patients were not considered to be clinically significant. No dose adjustment is recommended for elderly patients.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Fidaxomicin is a macrolide antibacterial drug for oral administration. Its CAS chemical name is Oxacyclooctadeca-3,5,9,13,15-pentaen-2-one, 3-[[[6-deoxy-4-O-(3,5-dichloro-2-ethyl-4,6-dihydroxybenzoyl)- 2-O-methyl-β-D-mannopyranosyl]oxy]methyl]-12-[[6-deoxy-5-C-methyl-4-O-(2-methyl-1-oxopropyl)-β-D- lyxo-hexopyranosyl]oxy]-11-ethyl-8-hydroxy-18-[(1R)-1-hydroxyethyl]-9,13,15-trimethyl-, (3E,5E,8S,9E,11S,12R,13E,15E,18S)-. The molecular formula is C52H74Cl2O18 and the molecular weight is 1058.04 g/mol. The structural formula of fidaxomicin is shown in Figure 1.
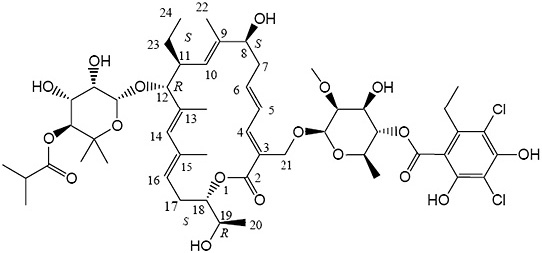
Figure 1: Structural Formula of Fidaxomicin
Fidaxomicin tablets are film-coated and contain 200 mg of fidaxomicin per tablet and the following inactive ingredients: butylated hydroxytoluene, hydroxypropyl cellulose, lecithin (soy), magnesium stearate, microcrystalline cellulose, polyethylene glycol 3350, polyvinyl alcohol-part hydrolyzed, pregelatinized corn starch, sodium starch glycolate Type A, talc, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Fidaxomicin acts locally in the gastrointestinal tract on C. difficile. In a dose-ranging trial (N=48) of fidaxomicin using 50 mg, 100 mg, and 200 mg twice daily for 10 days, a dose-response relationship was observed for efficacy.
12.3 Pharmacokinetics
The pharmacokinetic parameters of fidaxomicin and its main metabolite OP-1118 following a single dose of 200 mg in healthy adult males (N=14) are summarized in Table 4.
Table 4: Mean (± Standard Deviation) Pharmacokinetic Parameters of Fidaxomicin 200 mg in Healthy Adult Males Parameter
Fidaxomicin
OP-1118
N
Value
N
Value
Cmax (ng/mL)
14
5.20 ± 2.81
14
12.0 ± 6.06
Tmax (h)*
14
2.00 (1.00 to 5.00)
14
1.02 (1.00 to 5.00)
AUC0-t (ng-h/mL)
14
48.3 ± 18.4
14
103 ± 39.4
AUC0-∞ (ng-h/mL)
9
62.9 ± 19.5
10
118 ± 43.3
t1/2 (h)
9
11.7 ± 4.80
10
11.2 ± 3.01
* Tmax, reported as median (range).
Cmax, maximum observed concentration; Tmax, time to maximum observed concentration; AUC0-t, area under the concentration-time curve from time 0 to the last measured concentration; AUC0-∞, area under the concentration-time curve from time 0 to infinity; t1/2, elimination half-life
Absorption
Fidaxomicin has minimal systemic absorption following oral administration, with plasma concentrations of fidaxomicin and OP-1118 in the ng/mL range at the therapeutic dose. In fidaxomicin-treated patients from controlled trials, plasma concentrations of fidaxomicin and OP-1118 obtained within the Tmax window (1 to 5 hours) were approximately 2- to 6-fold higher than Cmax values in healthy adults. Following administration of fidaxomicin tablets 200 mg twice daily for 10 days, OP-1118 plasma concentrations within the Tmax window were approximately 50% to 80% higher than on Day 1, while concentrations of fidaxomicin were similar on Days 1 and 10.
In a food-effect study involving administration of fidaxomicin to healthy adults (N=28) with a high-fat meal versus under fasting conditions, Cmax of fidaxomicin and OP-1118 decreased by 21.5% and 33.4%, respectively, while AUC0-t remained unchanged. This decrease in Cmax is not considered clinically significant, and thus, fidaxomicin may be administered with or without food.
Distribution
Fidaxomicin is mainly confined to the gastrointestinal tract following oral administration. In selected patients (N=8) treated with fidaxomicin 200 mg twice daily for 10 days from controlled trials, fecal concentrations of fidaxomicin and OP-1118 obtained within 24 hours of the last dose ranged from 639 to 2710 mcg/g and 213 to 1210 mcg/g, respectively. In contrast, plasma concentrations of fidaxomicin and OP-1118 within the Tmax window (1 to 5 hours) ranged 2 to 179 ng/mL and 10 to 829 ng/mL, respectively.
Elimination
Metabolism
Fidaxomicin is primarily transformed by hydrolysis at the isobutyryl ester to form its main and microbiologically active metabolite, OP-1118. Metabolism of fidaxomicin and formation of OP-1118 are not dependent on cytochrome P450 (CYP) enzymes.
At the therapeutic dose, OP-1118 was the predominant circulating compound in healthy adults, followed by fidaxomicin.
Excretion
Fidaxomicin is mainly excreted in feces. In one trial of healthy adults (N=11), more than 92% of the dose was recovered in the stool as fidaxomicin and OP-1118 following single doses of 200 mg and 300 mg. In another trial of healthy adults (N=6), 0.59% of the dose was recovered in urine as OP-1118 only following a single dose of 200 mg.
Specific Populations
Geriatric Patients
In controlled trials of patients treated with fidaxomicin 200 mg twice daily for 10 days, mean and median values of fidaxomicin and OP-1118 plasma concentrations within the Tmax window (1 to 5 hours) were approximately 2- to 4-fold higher in elderly patients (≥65 years of age) versus non-elderly patients (<65 years of age). Despite greater exposures in elderly patients, fidaxomicin and OP-1118 plasma concentrations remained in the ng/mL range [see Use in Specific Populations (8.5)].
Pediatric Patients
Pediatric use information is approved for Cubist Pharmaceuticals LLC's DIFICID® (fidaxomicin) tablets. However, due to Cubist Pharmaceuticals LLC's marketing exclusivity rights, this drug product is not labeled with that information.
Male and Female Patients
Plasma concentrations of fidaxomicin and OP-1118 within the Tmax window (1 to 5 hours) did not vary by gender in patients treated with fidaxomicin 200 mg twice daily for 10 days from controlled trials. No dose adjustment is recommended based on gender.
Patients with Renal Impairment
In controlled trials of patients treated with fidaxomicin 200 mg twice daily for 10 days, plasma concentrations of fidaxomicin and OP-1118 within the Tmax window (1 to 5 hours) did not vary by severity of renal impairment (based on creatinine clearance) between mild (51 to 79 mL/min), moderate (31 to 50 mL/min), and severe (≤ 30 mL/min) categories. No dose adjustment is recommended based on renal function.
Patients with Hepatic Impairment
The impact of hepatic impairment on the pharmacokinetics of fidaxomicin has not been evaluated. Because fidaxomicin and OP-1118 do not appear to undergo significant hepatic metabolism, elimination of fidaxomicin and OP-1118 is not expected to be significantly affected by hepatic impairment.
Drug Interaction Studies
In vivo studies were conducted to evaluate intestinal drug-drug interactions of fidaxomicin as a P-gp substrate, P-gp inhibitor, and inhibitor of major CYP enzymes expressed in the gastrointestinal tract (CYP3A4, CYP2C9, and CYP2C19).
Table 5 summarizes the impact of a coadministered drug (P-gp inhibitor) on the pharmacokinetics of fidaxomicin [see Drug Interactions (7.1)].
Table 5: Pharmacokinetic Parameters of Fidaxomicin and OP-1118 in the Presence of a Coadministered Drug Parameter
Cyclosporine 200 mg + Fidaxomicin
200 mg* (N=14)
Fidaxomicin 200 mg Alone
(N=14)
Mean Ratio of Parameters With/Without Coadministered Drug (90% CI†) No Effect = 1.00
N
Mean
N
Mean
Fidaxomicin
Cmax (ng/mL)
14
19.4
14
4.67
4.15 (3.23 to 5.32)
AUC0-∞ (ng-h/mL)
8
114
9
59.5
1.92 (1.39 to 2.64)
OP-1118
Cmax (ng/mL)
14
100
14
10.6
9.51 (6.93 to 13.05)
AUC0-∞ (ng-h/mL)
12
438
10
106
4.11 (3.06 to 5.53)
* Cyclosporine was administered 1 hour before fidaxomicin.
† CI - confidence interval
Fidaxomicin had no significant impact on the pharmacokinetics of the following coadministered drugs: digoxin (P-gp substrate), midazolam (CYP3A4 substrate), warfarin (CYP2C9 substrate), and omeprazole (CYP2C19 substrate). No dose adjustment is warranted when fidaxomicin is coadministered with substrates of P-gp or CYP enzymes.
12.4 Microbiology
Mechanism of Action
Fidaxomicin is a fermentation product obtained from the Actinomycete Dactylosporangium aurantiacum. Fidaxomicin is a macrolide antibacterial drug that inhibits RNA synthesis by binding to RNA polymerases. Fidaxomicin is bactericidal against C. difficile in vitro, and demonstrates a post-antibiotic effect vs. C. difficile of 6 to 10 hrs.
Resistance
Fidaxomicin demonstrates no in vitro cross-resistance with other classes of antibacterial drugs. In vitro studies indicate a low frequency of spontaneous resistance to fidaxomicin in C. difficile (ranging from <1.4 × 10-9 to 12.8 × 10-9). A specific mutation (Val-ll43-Gly) in the beta subunit of RNA polymerase is associated with reduced susceptibility to fidaxomicin. This mutation was created in the laboratory and seen during clinical trials in a C. difficile isolate obtained from an adult subject treated with fidaxomicin who had recurrence of CDAD. The fidaxomicin minimum inhibitory concentration (MIC) of the C. difficile isolate from this subject increased from a baseline of 0.06 mcg/mL to 16 mcg/mL at the time of CDAD recurrence.
Interaction with Other Antimicrobials
Fidaxomicin and its main metabolite OP-1118 do not exhibit any antagonistic interaction with other classes of antibacterial drugs. Synergistic interactions of fidaxomicin and OP-1118 have been observed in vitro with rifampin and rifaximin against C. difficile.
Antimicrobial Activity
Fidaxomicin has been shown to be active against most isolates of Clostridioides (formerly Clostridium) difficile, both in vitro and in clinical infections [see Indications and Usage (1)].
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria, and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies have not been conducted to evaluate the carcinogenic potential of fidaxomicin.
Neither fidaxomicin nor OP-1118 was mutagenic in the Ames assay. Fidaxomicin was also negative in the rat micronucleus assay. However, fidaxomicin was clastogenic in Chinese hamster ovary cells.
Fidaxomicin did not affect the fertility of male and female rats at intravenous doses of 6.3 mg/kg. The exposure (AUC0-t) was approximately 100 times that in humans.
-
14 CLINICAL STUDIES
14.1 Clinical Studies of Fidaxomicin in Adult Patients with CDAD
In two randomized, double-blinded trials, a non-inferiority design was utilized to demonstrate the efficacy of fidaxomicin (200 mg tablets twice daily for 10 days) compared to vancomycin (125 mg four times daily for 10 days) in adults with CDAD.
Enrolled patients were 18 years of age or older and received no more than 24 hours of pretreatment with vancomycin or metronidazole. CDAD was defined by >3 unformed bowel movements (or >200 mL of unformed stool for subjects having rectal collection devices) in the 24 hours before randomization, and presence of either C. difficile toxin A or B in the stool within 48 hours of randomization. Enrolled patients had either no prior CDAD history or only one prior CDAD episode in the past three months. Subjects with life-threatening/fulminant infection, hypotension, septic shock, peritoneal signs, significant dehydration, or toxic megacolon were excluded.
The demographic profile and baseline CDAD characteristics of enrolled subjects were similar in the two trials. Patients had a median age of 64 years, were mainly white (90%), female (58%), and inpatients (63%). The median number of bowel movements per day was 6, and 37% of subjects had severe CDAD (defined as 10 or more unformed bowel movements per day or WBC ≥15000/mm3). Diarrhea alone was reported in 45% of patients and 84% of subjects had no prior CDAD episode.
The primary efficacy endpoint was the clinical response rate at the end of treatment, based upon improvement in diarrhea or other symptoms such that, in the investigator's judgment, further CDAD treatment was not needed. An additional efficacy endpoint was sustained clinical response 25 days after the end of treatment. Sustained response was evaluated only for patients who were clinical successes at the end of treatment. Sustained response was defined as clinical response at the end of treatment, and survival without proven or suspected CDAD recurrence through 25 days beyond the end of treatment.
The results for clinical response at the end of treatment in both trials, shown in Table 6, indicate that fidaxomicin is non-inferior to vancomycin based on the 95% confidence interval (CI) lower limit being greater than the non-inferiority margin of -10%.
The results for sustained clinical response at the end of the follow-up period, also shown in Table 6, indicate that fidaxomicin is superior to vancomycin on this endpoint. Since clinical success at the end of treatment and mortality rates were similar across treatment arms (approximately 6% in each group), differences in sustained clinical response were due to lower rates of proven or suspected CDAD during the follow-up period in fidaxomicin patients.
Table 6: Clinical Response Rates at End-of-Treatment and Sustained Response at 25 days Post-Treatment in Adult Patients
Clinical Response at End of Treatment
Sustained Response at 25 days Post-Treatment
Fidaxomicin
% (N)
Vancomycin
% (N)
Difference
(95% CI)*
Fidaxomicin
% (N)
Vancomycin
% (N)
Difference
(95% CI)*
Trial 1
88%
(N=289)86%
(N=307)2.6%
(-2.9%, 8.0%)70%
(N=289)57%
(N=307)12.7%
(4.4%, 20.9%)Trial 2
88%
(N=253)87%
(N=256)1.0%
(-4.8%, 6.8%)72%
(N=253)57%
(N=256)14.6%
(5.8%, 23.3%)* Confidence interval (CI) was derived using Wilson's score method. Approximately 5%-9% of the data in each trial and treatment arm were missing sustained response information and were imputed using multiple imputation method.
Restriction Endonuclease Analysis (REA) was used to identify C. difficile baseline isolates in the BI group, isolates associated with increasing rates and severity of CDAD in the US in the years prior to the clinical trials. Similar rates of clinical response at the end of treatment and proven or suspected CDAD during the follow-up period were seen in fidaxomicin-treated and vancomycin-treated patients infected with a BI isolate. However, fidaxomicin did not demonstrate superiority in sustained clinical response when compared with vancomycin (Table 7).
Table 7: Sustained Clinical Response at 25 Days after Treatment by C. difficile REA Group at Baseline in Adult Patients Trial 1
Initial C. difficile Group
Fidaxomicin
n/N (%)
Vancomycin
n/N (%)
Difference
(95% CI)*
BI Isolates
44/76 (58%)
52/82 (63%)
-5.5% (-20.3%, 9.5%)
Non-BI Isolates
105/126 (83%)
87/131 (66%)
16.9% (6.3%, 27.0%)
Trial 2
Initial C. difficile Group
Fidaxomicin
n/N (%)
Vancomycin
n/N (%)
Difference
(95% CI)*
BI Isolates
42/65 (65%)
31/60 (52%)
12.9% (-4.2%, 29.2%)
Non-BI Isolates
109/131 (83%)
77/121 (64%)
19.6% (8.7%, 30.0%)
* Interaction test between the effect on sustained response rate and BI versus non-BI isolates using logistic regression (p-values: trial 1: 0.009; trial 2: 0.29). Approximately 25% of the mITT population were missing data for REA group. Confidence intervals (CI) were derived using Wilson's score method.
Pediatric use information is approved for Cubist Pharmaceuticals LLC's DIFICID® (fidaxomicin) tablets. However, due to Cubist Pharmaceuticals LLC's marketing exclusivity rights, this drug product is not labeled with that information.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Fidaxomicin tablets are supplied as follows:
200 mg – Each white, film-coated, modified capsule shape tablet, debossed with A205 on one side and plain on the other side contains 200 mg of fidaxomicin. Tablets are supplied as bottles of 20 tablets NDC: 0480-2596-34.
Store in the original bottle.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Administration with Food
Inform patients and caregivers that fidaxomicin tablets may be taken with or without food.
Antibacterial Resistance
Patients should be counseled that antibacterial drugs, including fidaxomicin tablets, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When fidaxomicin tablets are prescribed to treat a C. difficile infection, patients should be told that, although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by fidaxomicin tablets or other antibacterial drugs in the future.
Manufactured For:
Teva Pharmaceuticals
Parsippany, NJ 07054Iss. 5/2024
-
PATIENT INFORMATION
Fidaxomicin (fye dax" oh mye' sin)
Tablets, for oral use
What You Need to Know About Your Medicine
- Before you take fidaxomicin tablets, be sure you understand what it is for and how to take it.
- If you have questions about fidaxomicin tablets, ask your doctor or pharmacist.
- Remember that your doctor has prescribed fidaxomicin tablets only for you. Never give this medicine to anyone else.
- Keep this Patient Information for fidaxomicin tablets so you can read it again.
What are fidaxomicin tablets?
Fidaxomicin tablets are an antibiotic medicine used to treat an infection called Clostridioides difficile-associated diarrhea (CDAD) in adults. Clostridioides difficile (C-diff) is a bacterium that can cause an infection that can damage your colon and cause stomach pain and severe diarrhea.
- Fidaxomicin tablets are not to be used to treat other types of infections in the body.
- Sometimes infections are caused by viruses rather than bacteria. Antibiotic medicines, including fidaxomicin tablets, do not kill viruses.
It is not known if fidaxomicin tablets are safe and effective in children under 6 months old.
Who should not take fidaxomicin tablets?
Do not take fidaxomicin tablets if you are allergic to fidaxomicin, or any other ingredient in fidaxomicin tablets. See the end of this Patient Information for a complete list of ingredients in fidaxomicin tablets.
What should I tell my doctor before taking fidaxomicin tablets?
Pregnancy
- If you are pregnant or plan to become pregnant, tell your doctor before you take fidaxomicin tablets.
- It is not known if fidaxomicin tablets will harm your baby while you are pregnant.
- If you are pregnant, you and your doctor should decide together if you will take fidaxomicin tablets.
Breastfeeding
- If you are breastfeeding or plan to breastfeed, tell your doctor before you take fidaxomicin tablets.
- It is not known if fidaxomicin passes into breast milk.
- If you are breastfeeding, you and your doctor should decide together if you will take fidaxomicin tablets.
Other Medicines
- Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal and dietary supplements.
- Know the medicines you take. Keep a list of your medicines to show your doctor and pharmacist when you get a new medicine.
Allergic Reactions
- See “Who should not take fidaxomicin tablets?”
- If you are allergic to other kinds of antibiotics called macrolides (for example: azithromycin (Zithromax®) or clarithromycin (Biaxin®)) or any other ingredient in fidaxomicin tablets, tell your doctor. See the end of this Patient Information for a complete list of ingredients in fidaxomicin tablets.
How do I take fidaxomicin tablets?
- Take fidaxomicin tablets exactly as prescribed by your doctor.
- Take fidaxomicin tablets twice a day (approximately every 12 hours). For example, if you take your first dose at 8:00 a.m. you should take your second dose at 8:00 p.m.
- You can take fidaxomicin tablets with or without food.
- Do not skip any doses or stop taking fidaxomicin tablets until you finish your prescribed treatment, even if you begin to feel better, unless you have a serious allergic reaction (see “What are the possible side effects of fidaxomicin tablets?”).
This will lower the chance that the bacteria will become resistant to fidaxomicin tablets. If this happens, fidaxomicin tablets and other antibiotic medicines may not work in the future.
What are the possible side effects of fidaxomicin tablets?
Fidaxomicin tablets can cause serious side effects, including:
- Allergic reaction. If you get a severe allergic reaction while taking fidaxomicin tablets, including problems breathing or shortness of breath, rash, itching or hives, or swelling of the mouth, throat, or face, stop taking fidaxomicin tablets and get emergency medical help right away.
Common side effects of fidaxomicin tablets include:
The most common side effects of fidaxomicin tablets in adults include:
- nausea
- vomiting
- stomach pain
- bleeding in the stomach or intestines
- low red blood cell count (anemia)
- low white blood cell count (neutropenia)
Other less common side effects of fidaxomicin tablets may include:
- swelling of any body part (such as your face, lips, tongue or around your eyes)
- itching
- hives
- bloating
- stomach tenderness
- heartburn
- problems swallowing
- high blood sugar (hyperglycemia)
- abnormal liver tests
- low levels of blood bicarbonate
- passing gas
- intestinal blockage
- serious bowel inflammation (toxic megacolon)
- low platelet count (important for clotting and to control bleeding)
- high levels of acid in your blood (metabolic acidosis)
If you have any side effect that bothers you or does not go away, tell your doctor.
There may be other side effects to fidaxomicin tablets that are not listed. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store fidaxomicin tablets?
- Store fidaxomicin tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep fidaxomicin tablets in its original bottle until you are ready to take it.
Keep fidaxomicin tablets and all medicines out of the reach of children.
General information about the safe and effective use of fidaxomicin tablets.
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information. Do not take fidaxomicin tablets for a condition for which they were not prescribed. Do not give fidaxomicin tablets to other people, even if they have the same symptoms that you have. They may harm them. You can ask your pharmacist or doctor for information about fidaxomicin tablets that is written for health professionals.
What if I have questions?
- Call your doctor.
- Call Teva at 1-888-838-2872.
What are the ingredients in fidaxomicin tablets?
Active ingredient: fidaxomicin.
Inactive ingredients: butylated hydroxytoluene, hydroxypropyl cellulose, lecithin (soy), magnesium stearate, microcrystalline cellulose, polyethylene glycol 3350, polyvinyl alcohol-part hydrolyzed, pregelatinized corn starch, sodium starch glycolate Type A, talc, and titanium dioxide.Pediatric use information is approved for Cubist Pharmaceuticals LLC's DIFICID® (fidaxomicin) tablets. However, due to Cubist Pharmaceuticals LLC's marketing exclusivity rights, this drug product is not labeled with that information.
Brands listed are the trademarks of their respective owners.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured For:
Teva Pharmaceuticals
Parsippany, NJ 07054Iss. 5/2024
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
FIDAXOMICIN
fidaxomicin tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0480-2596 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FIDAXOMICIN (UNII: Z5N076G8YQ) (FIDAXOMICIN - UNII:Z5N076G8YQ) FIDAXOMICIN 200 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape OVAL (modified capsule) Size 14mm Flavor Imprint Code A205 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0480-2596-34 20 in 1 BOTTLE; Type 0: Not a Combination Product 07/15/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208443 07/15/2025 Labeler - Teva Pharmaceuticals, Inc. (022629579)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.