Dayclear allergy releif 8oz
Dayclear Allergy Relief by
Drug Labeling and Warnings
Dayclear Allergy Relief by is a Otc medication manufactured, distributed, or labeled by GM Pharmaceuticals INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DAYCLEAR ALLERGY RELIEF- allergy releif liquid
GM Pharmaceuticals INC
----------
Dayclear allergy releif 8oz
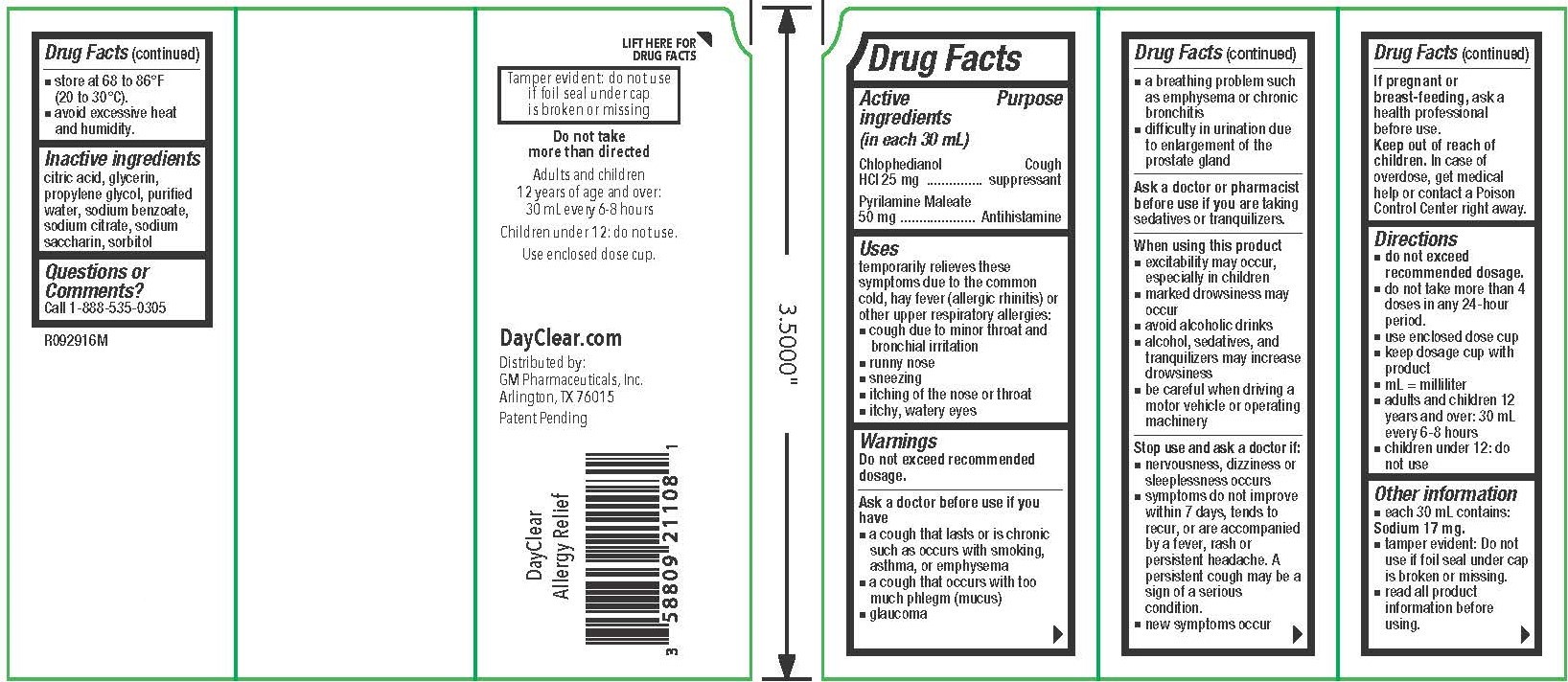
Active Purpose
ingredients
(in each 30 mL)
Chlophedianol Cough
HCl 25 mg ............... suppressant
Pyrilamine Maleate
50 mg .................... Antihistamine
temporarily relieves these
symptoms due to the common
cold, hay fever (allergic rhinitis) or
other upper respiratory allergies:
▪ cough due to minor throat and
bronchial irritation
▪ runny nose
▪ sneezing
▪ itching of the nose or throat
▪ itchy, watery eyes
▪ a cough that lasts or is chronic
such as occurs with smoking,
asthma, or emphysema
▪ a cough that occurs with too
much phlegm (mucus)
▪ glaucoma
▪ a breathing problem such
as emphysema or chronic
bronchitis
▪ difficulty in urination due
to enlargement of the
prostate gland
▪ do not exceed
recommended dosage.
▪ do not take more than 4
doses in any 24-hour
period.
▪ use enclosed dose cup
▪ keep dosage cup with
product
▪ mL = milliliter
▪ adults and children 12
years and over: 30 mL
every 6-8 hours
▪ children under 12: do
not use
each 30 mL contains:
Sodium 17 mg.
▪
tamper evident: Do not
use if foil seal under cap
is broken or missing.
▪ read all product
information before
using.
citric acid, glycerin, propylene glycol, purified water, sodium benzoate, sodium citrate, sodium saccharin, sorbitol
| DAYCLEAR ALLERGY RELIEF
allergy releif liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GM Pharmaceuticals INC (793000860) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.