ChigaRid by Colgin Inc ChigaRid
ChigaRid by
Drug Labeling and Warnings
ChigaRid by is a Otc medication manufactured, distributed, or labeled by Colgin Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
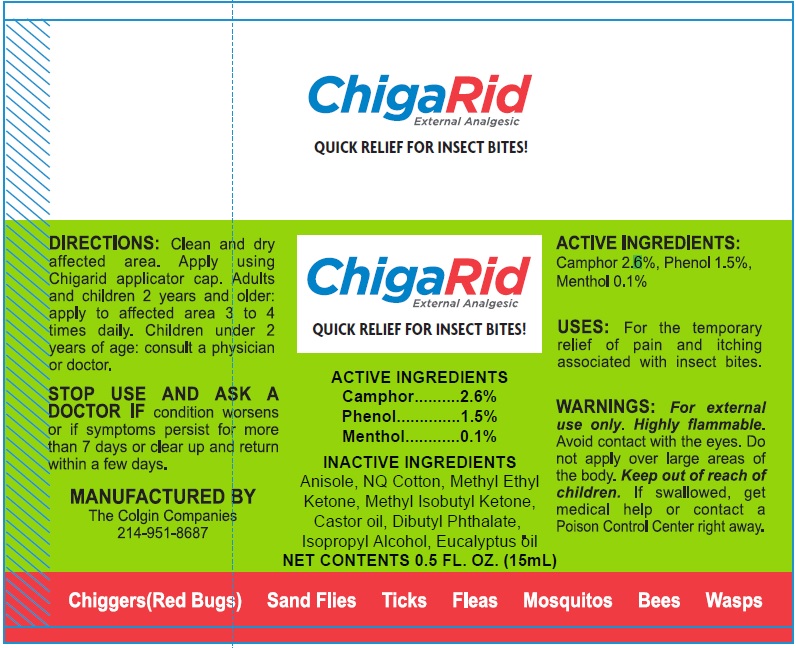
CHIGARID- camphor (synthetic), phenol, menthol solutionÂ
Colgin Inc
----------
ChigaRid
WARNINGS:
Avoid contact with the eyes. Do not apply over large areas of the body. For external use only. Highly flammable.
DIRECTIONS:
Clean and dry affected area. Apply using Chigarid applicator cap. Adults and children 2 years and older: apply to affected area 3 to 4 times daily. Children under 2 years of age: consult a physician or doctor.
| CHIGARIDÂ
camphor (synthetic), phenol, menthol solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler -Â Colgin Inc (799552443) |
Revised: 10/2024
Â
Document Id: 2483acec-42ed-617a-e063-6294a90a4963
Set id: dfed19af-75d5-4daa-b61f-e7e3bf680fb6
Version: 5
Effective Time: 20241015
Trademark Results [ChigaRid]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CHIGARID 88345255 5863063 Live/Registered |
Colgin, Inc. 2019-03-18 |
 CHIGARID 75917367 2429817 Dead/Cancelled |
COLGIN COMPANIES, THE 2000-02-14 |
 CHIGARID 71577512 0563195 Dead/Expired |
RICHARD COLGIN CO., INC. 1949-04-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.