Cold and Allergy by Care One (American Sales Company) DRUG FACTS
Cold and Allergy by
Drug Labeling and Warnings
Cold and Allergy by is a Otc medication manufactured, distributed, or labeled by Care One (American Sales Company). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
COLD AND ALLERGY- chlorpheniramine maleate, phenylephrine hcl tablet
Care One (American Sales Company)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
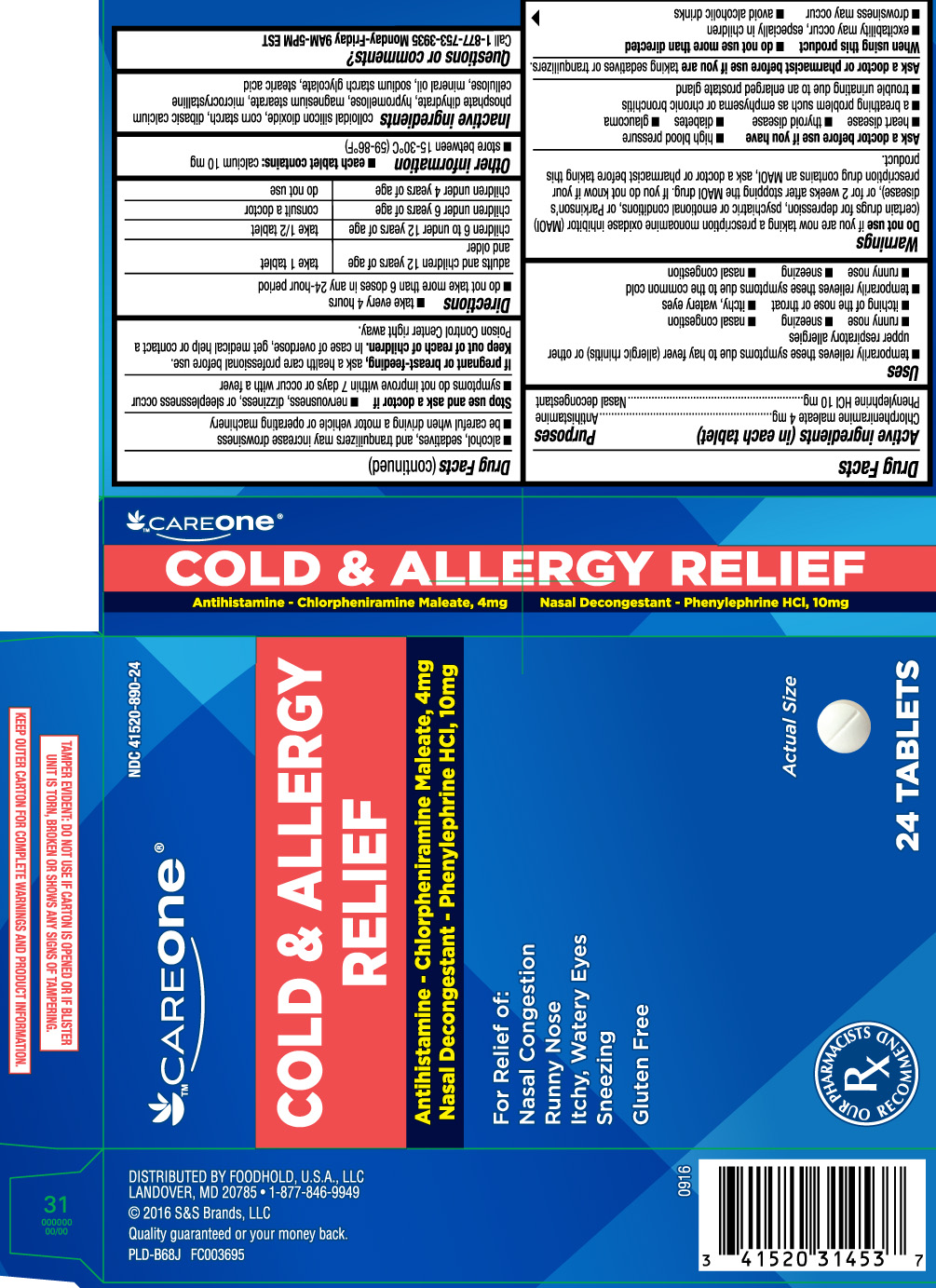
DRUG FACTS
Uses
- temporarily relieves these symptoms due to hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- nasal congestion
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
- nasal congestion
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- high blood pressure
- heart disease
- thyroid disease
- diabetes
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
- excitability may occur, especially in children
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Directions
- take every 4 hours
- do not take more than 6 doses in 24 hours
| adults and children 12 years of age and older | take 1 tablet |
| children 6 to under 12 years of age | take 1/2 tablet |
| children under 6 years of age | consult a doctor |
| children under 4 years of age | do not use |
Inactive ingredients
colloidal silicon dioxide, corn starch,dibasic calcium phosphate dihydrate, hypromellose, magnesium stearate, microcrystalline cellulose, mineral oil, sodium starch glycolate and stearic acid.
Principal Display Panel
COLD & ALLERGY RELIEF
Antihistamine - Chlorpheniramine Maleate, 4 mg
Nasal Decongestant - Phenylephrine HCI, 10 mg
For Relief of:
Nasal Congestion
Runny Nose
Itchy, Watery Eyes
Sneezing
Gluten Free
TABLETS
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
DISTRIBUTED BY FOODHOLD, U.S.A., LLC
LANDOVER, MD 20785 1-877-846-9949
| COLD AND ALLERGY
chlorpheniramine maleate, phenylephrine hcl tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Care One (American Sales Company) (809183973) |