DIUREX WATER PILLS- caffeine, magnesium salicylate tablet, coated
Diurex by
Drug Labeling and Warnings
Diurex by is a Otc medication manufactured, distributed, or labeled by Alva-Amco Pharmacal Companies, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
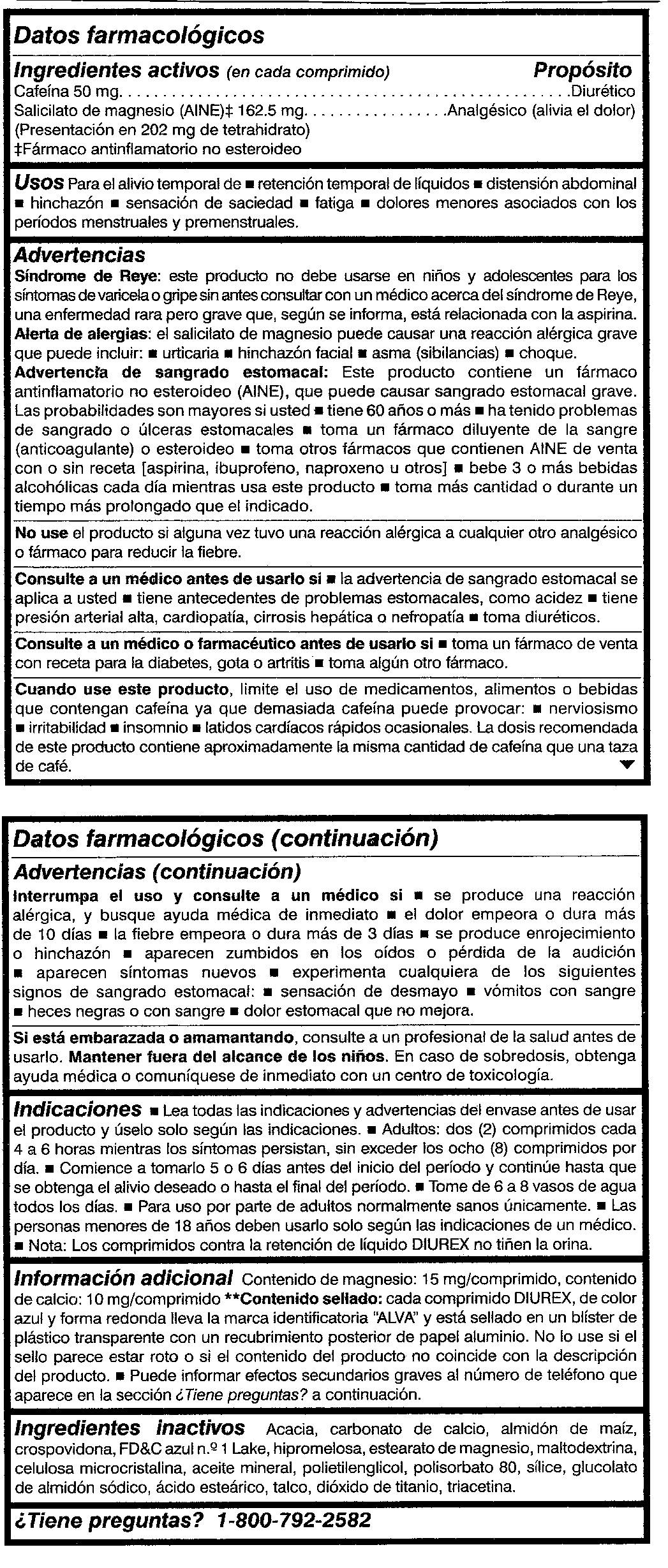
- Active ingredients (in each pill)
- Purpose
- Uses
-
Warnings
Reye's Syndrome: Children and teenagers should not use this product for chicken pox or flu symptoms before a doctor is consulted about Reye's Syndrome, a rare but serious illness reported to be associated with aspirin.
Allergy alert: Magnesium Salicylate may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning: This product contains a non-steroidal anti-inflammatory drug (NSAID), which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen or others]
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed.
- DO NOT USE
- Ask a doctor before use if
- Ask a doctor or pharmacist before use if you are
- WHEN USING
-
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- pain gets worse or lasts more than 10 days.
- fever gets worse or lasts more than 3 days.
- redness or swelling is present.
- ringing in the ears or loss of hearing occurs.
- any new symptoms appear.
- you experience any of the following signs of stomach bleeding:
-
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better.
- feel faint
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Read all package directions and warnings before use and use only as directed.
- Adults: Two (2) pills every 4 to 6 hours while symptoms persist, not to exceed eight (8) pills per day.
- Start taking 5 or 6 days before onset of period and continue until desired relief is obtained or end of period.
- Drink 6 to 8 glasses of water daily.
- For use by normally healthy adults only.
- Persons under 18 years of age should use only as directed by a doctor.
- Note: DIUREX Water Pills will not discolor urine.
-
Other information
Magnesium content: 15 mg/pill, Calcium content: 10 mg/pill **Contents sealed: Each DIUREX blue colored, round shaped pill bears the identifying mark “ALVA" and is sealed in a clear plastic blister with a foil backing. Do not use if seal appears broken or if product contents do not match product description. You may report serious side effects to the phone number provided under Questions? below.
- Inactive ingredients
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIUREX WATER PILLS
caffeine, magnesium salicylate tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 52389-306 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM SALICYLATE (UNII: 41728CY7UX) (SALICYLIC ACID - UNII:O414PZ4LPZ) MAGNESIUM SALICYLATE 162.5 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 50 mg Inactive Ingredients Ingredient Name Strength ACACIA (UNII: 5C5403N26O) CALCIUM CARBONATE (UNII: H0G9379FGK) STARCH, CORN (UNII: O8232NY3SJ) CROSPOVIDONE (UNII: 2S7830E561) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color blue Score no score Shape ROUND Size 10mm Flavor Imprint Code ALVA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52389-306-84 2 in 1 CARTON 05/02/2012 1 NDC: 52389-306-01 42 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 52389-306-42 1 in 1 CARTON 05/15/2005 2 NDC: 52389-306-01 42 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 52389-306-22 2 in 1 CARTON 05/15/2005 11/30/2018 3 NDC: 52389-306-02 11 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC: 52389-306-21 2 in 1 CARTON 09/15/2014 10/31/2018 4 21 in 1 BLISTER PACK; Type 0: Not a Combination Product 5 NDC: 52389-306-36 2 in 1 CARTON 02/14/2020 5 18 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 05/15/2005 Labeler - Alva-Amco Pharmacal Companies, Inc. (042074856)
Trademark Results [Diurex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DIUREX 97446554 not registered Live/Pending |
Alva-Amco Pharmacal Companies, LLC. 2022-06-07 |
 DIUREX 75656624 2415172 Live/Registered |
Alva-Amco Pharmacal Cos., Inc. 1999-03-09 |
 DIUREX 73011429 1001952 Dead/Expired |
GERCHENSON, EMILE H. 1974-01-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.