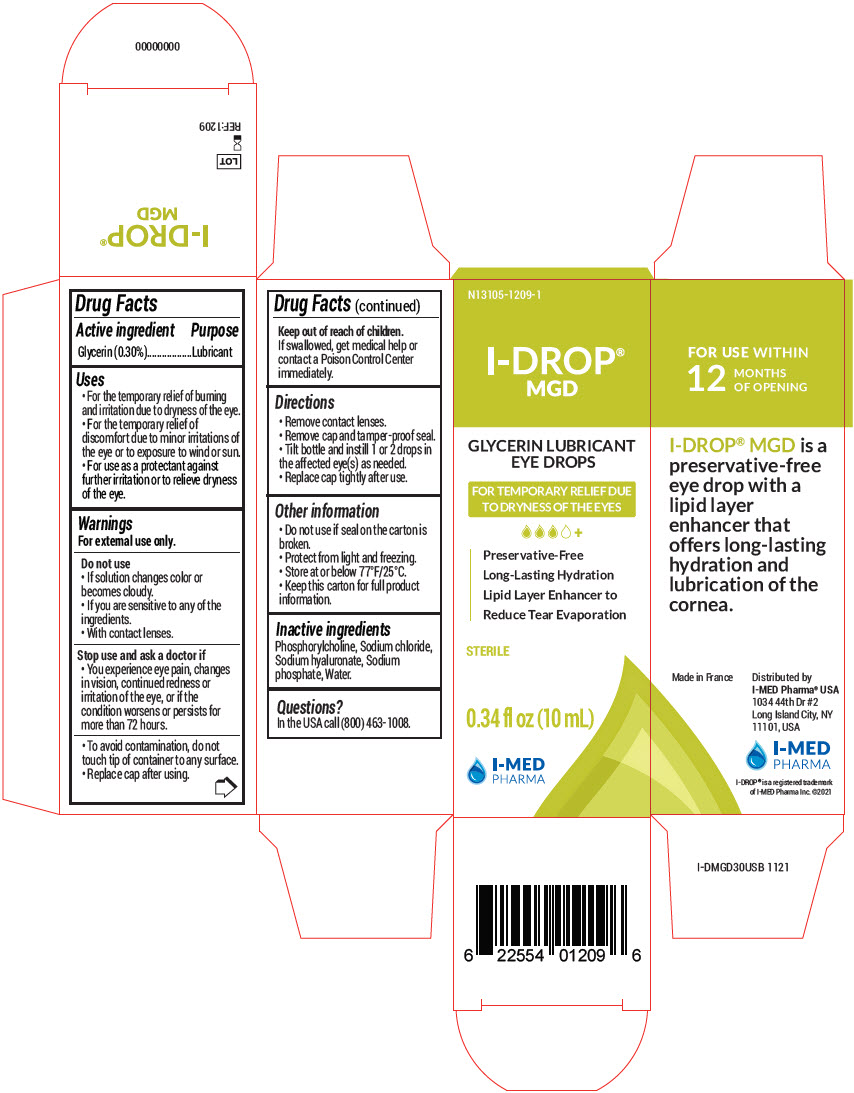
I-Drop MGD by I-MED PHARMA I-Drop® MGD
I-Drop MGD by
Drug Labeling and Warnings
I-Drop MGD by is a Otc medication manufactured, distributed, or labeled by I-MED PHARMA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
I-DROP MGD- glycerin liquid
I-MED PHARMA
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
I-Drop® MGD
Uses
- For the temporary relief of burning and irritation due to dryness of the eye.
- For the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun.
- For use as a protectant against further irritation or to relieve dryness of the eye.
Warnings
For external use only.
Do not use
- If solution changes color or becomes cloudy.
- If you are sensitive to any of the ingredients.
- With contact lenses.
Directions
- Remove contact lenses.
- Remove cap and tamper-proof seal.
- Tilt bottle and instill 1 or 2 drops in the affected eye(s) as needed.
- Replace cap tightly after use.
Other information
- Do not use if seal on the carton is broken.
- Protect from light and freezing.
- Store at or below 77°F/25°C.
- Keep this carton for full product information.
| I-DROP MGD
glycerin liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - I-MED PHARMA (249008772) |
Revised: 8/2022
<
Document Id: c6f1ecea-d0d4-48a9-aecc-be7c63f53053
Set id: e02e7992-cc07-45aa-b1c9-f84411ca123c
Version: 2
Effective Time: 20220816
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.