POSACONAZOLE tablet, coated
POSACONAZOLE by
Drug Labeling and Warnings
POSACONAZOLE by is a Prescription medication manufactured, distributed, or labeled by Par Pharmaceutical Inc., Merck Sharp & Dohme LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use POSACONAZOLE delayed-release tablets safely and effectively. See full prescribing information for POSACONAZOLE delayed-release tablets.
Posaconazole delayed-release tablets, for oral use
Initial U.S. Approval: 2006INDICATIONS AND USAGE
Posaconazole is an azole antifungal agent indicated for:
delayed-release tablets and oral suspension- prophylaxis of invasive Aspergillus and Candida infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as HSCT recipients with GVHD or those with hematologic malignancies with prolonged neutropenia from chemotherapy. (1.1)
DOSAGE AND ADMINISTRATION
Posaconazole delayed-release tablets and oral suspension are not interchangeable due to the differences in the dosing of each formulation. Therefore, follow the specific dosage recommendations for each of the formulations. (2.2, 2.3)
Indication Dose and Duration of Therapy - * Posaconazole delayed-release tablets should be taken with food. (2)
Prophylaxis of invasive Aspergillus and Candida infections Delayed-Release Tablets*:
Loading dose: 300 mg (three 100 mg delayed-release tablets) twice a day on the first day.
Maintenance dose: 300 mg (three 100 mg delayed-release tablets) once a day, starting on the second day. Duration of therapy is based on recovery from neutropenia or immunosuppression. (2.2)DOSAGE FORMS AND STRENGTHS
- Posaconazole delayed-release tablet 100 mg (3)
CONTRAINDICATIONS
- Do not administer to persons with known hypersensitivity to posaconazole or other azole antifungal agents. (4.1)
- Do not coadminister posaconazole with the following drugs; Posaconazole increases concentrations of:
- Sirolimus: can result in sirolimus toxicity (4.2, 7.1)
- CYP3A4 substrates (pimozide, quinidine): can result in QTc interval prolongation and cases of TdP (4.3, 7.2)
- HMG-CoA Reductase Inhibitors Primarily Metabolized Through CYP3A4: can lead to rhabdomyolysis (4.4, 7.3)
- Ergot alkaloids: can result in ergotism (4.5, 7.4)
WARNINGS AND PRECAUTIONS
- Calcineurin-Inhibitor Toxicity: Posaconazole increases concentrations of cyclosporine or tacrolimus; reduce dose of cyclosporine and tacrolimus and monitor concentrations frequently. (5.1)
- Arrhythmias and QTc Prolongation: Posaconazole has been shown to prolong the QTc interval and cause cases of TdP. Administer with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs known to prolong QTc interval and metabolized through CYP3A4. (5.2)
- Electrolyte Disturbances: Monitor and correct, especially those involving potassium (K+), magnesium (Mg++), and calcium (Ca++), before and during posaconazole therapy. (5.3)
- Hepatic Toxicity: Elevations in LFTs may occur. Discontinuation should be considered in patients who develop abnormal LFTs or monitor LFTs during treatment. (5.4)
- Midazolam: Posaconazole can prolong hypnotic/sedative effects. Monitor patients and benzodiazepine receptor antagonists should be available. (5.6, 7.5)
- Vincristine Toxicity: Concomitant administration of azole antifungals, including Posaconazole, with vincristine has been associated with neurotoxicity and other serious adverse reactions; reserve azole antifungals, including posaconazole, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options. (5.7, 7.10)
ADVERSE REACTIONS
- Common treatment-emergent adverse reactions in studies with posaconazole are diarrhea, nausea, fever, vomiting, headache, coughing, and hypokalemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Interaction Drug Interaction - * The drug interactions with esomeprazole and metoclopramide do not apply to posaconazole tablets.
Rifabutin, phenytoin, efavirenz, cimetidine, esomeprazole* Avoid coadministration unless the benefit outweighs the risks (7.6, 7.7, 7.8, 7.9) Other drugs metabolized by CYP3A4 Consider dosage adjustment and monitor for adverse effects and toxicity (7.1, 7.10, 7.11) Digoxin Monitor digoxin plasma concentrations (7.12) Fosamprenavir, metoclopramide* Monitor for breakthrough fungal infections (7.6, 7.13) USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Invasive Aspergillus and Candida Infections
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions for Posaconazole Delayed-Release Tablets
2.2 Dosage and Administration Instructions for Posaconazole Delayed-Release Tablets
2.3 Non-Interchangeability between Posaconazole Delayed-Release Tablets and Posaconazole Oral Suspension
2.4 Dosage Adjustments in Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Use with Sirolimus
4.3 QT Prolongation with Concomitant Use with CYP3A4 Substrates
4.4 HMG-CoA Reductase Inhibitors Primarily Metabolized Through CYP3A4
4.5 Use with Ergot Alkaloids
5 WARNINGS AND PRECAUTIONS
5.1 Calcineurin-Inhibitor Drug Interactions
5.2 Arrhythmias and QT Prolongation
5.3 Electrolyte Disturbances
5.4 Hepatic Toxicity
5.5 Renal Impairment
5.6 Use with Midazolam
5.7 Vincristine Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Immunosuppressants Metabolized by CYP3A4
7.2 CYP3A4 Substrates
7.3 HMG-CoA Reductase Inhibitors (Statins) Primarily Metabolized Through CYP3A4
7.4 Ergot Alkaloids
7.5 Benzodiazepines Metabolized by CYP3A4
7.6 Anti-HIV Drugs
7.7 Rifabutin
7.8 Phenytoin
7.9 Gastric Acid Suppressors/Neutralizers
7.10 Vinca Alkaloids
7.11 Calcium Channel Blockers Metabolized by CYP3A4
7.12 Digoxin
7.13 Gastrointestinal Motility Agents
7.14 Glipizide
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Gender
8.9 Race
8.10 Weight
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Prophylaxis of Aspergillus and Candida Infections with Posaconazole Oral Suspension
14.2 Treatment of Oropharyngeal Candidiasis with Posaconazole Oral Suspension
14.3 Posaconazole Oral Suspension Treatment of Oropharyngeal Candidiasis Refractory to Treatment with Fluconazole or Itraconazole
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Administration
17.2 Drug Interactions
17.3 Serious and Potentially Serious Adverse Reactions
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Invasive Aspergillus and Candida Infections
Posaconazole delayed-release tablets and oral suspension are indicated for prophylaxis of invasive Aspergillus and Candida infections in patients who are at high risk of developing these infections due to being severely immunocompromised, such as hematopoietic stem cell transplant (HSCT) recipients with graft-versus-host disease (GVHD) or those with hematologic malignancies with prolonged neutropenia from chemotherapy.
Posaconazole delayed-release tablets are indicated in patients 13 years of age and older.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions for Posaconazole Delayed-Release Tablets
Posaconazole delayed-release tablets and oral suspension are not to be used interchangeably due to the differences in the dosing of each formulation [see Dosage and Administration (2.2, 2.3, 2.4)].
Posaconazole delayed-release tablets
- Swallow tablets whole. Do not divide, crush, or chew.
- Administer with food [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
- Patients who have severe diarrhea or vomiting should be monitored closely for breakthrough fungal infections when receiving posaconazole delayed-release tablets.
2.2 Dosage and Administration Instructions for Posaconazole Delayed-Release Tablets
Dosage:
Table 1: Dosage for Posaconazole Delayed-Release Tablets Indication Dose and Duration of Therapy Prophylaxis of invasive Aspergillus and Candida infections Loading dose: 300 mg (three 100 mg delayed-release tablets) twice a day on the first day.
Maintenance dose: 300 mg (three 100 mg delayed-release tablets) once a day, starting on the second day. Duration of therapy is based on recovery from neutropenia or immunosuppression.Administration Instructions for Posaconazole Delayed-Release Tablets:
- Swallow tablets whole. Do not divide, crush, or chew.
- Administer posaconazole delayed-release tablets with food to enhance the oral absorption of posaconazole and optimize plasma concentrations [see Clinical Pharmacology (12.3)].
- Posaconazole delayed-release tablets should be used only for the prophylaxis indication.
- Posaconazole delayed-release tablets generally provide higher plasma drug exposures than posaconazole oral suspension under both fed and fasted conditions, and therefore is the preferred oral formulation for the prophylaxis indication.
2.3 Non-Interchangeability between Posaconazole Delayed-Release Tablets and Posaconazole Oral Suspension
Posaconazole delayed-release tablets and oral suspension are not to be used interchangeably due to the differences in the dosing of each formulation. Therefore, follow the specific dosage recommendations for each of the formulations [see Dosage and Administration (2.2, 2.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Posaconazole is contraindicated in persons with known hypersensitivity to posaconazole or other azole antifungal agents.
4.2 Use with Sirolimus
Posaconazole is contraindicated with sirolimus. Concomitant administration of posaconazole with sirolimus increases the sirolimus blood concentrations by approximately 9-fold and can result in sirolimus toxicity [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
4.3 QT Prolongation with Concomitant Use with CYP3A4 Substrates
Posaconazole is contraindicated with CYP3A4 substrates that prolong the QT interval. Concomitant administration of posaconazole with the CYP3A4 substrates, pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes [see Warnings and Precautions (5.2) and Drug Interactions (7.2)].
4.4 HMG-CoA Reductase Inhibitors Primarily Metabolized Through CYP3A4
Coadministration with the HMG-CoA reductase inhibitors that are primarily metabolized through CYP3A4 (e.g., atorvastatin, lovastatin, and simvastatin) is contraindicated since increased plasma concentration of these drugs can lead to rhabdomyolysis [see Drug Interactions (7.3) and Clinical Pharmacology (12.3)].
4.5 Use with Ergot Alkaloids
Posaconazole may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism [see Drug Interactions (7.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Calcineurin-Inhibitor Drug Interactions
Concomitant administration of posaconazole with cyclosporine or tacrolimus increases the whole blood trough concentrations of these calcineurin-inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. Nephrotoxicity and leukoencephalopathy (including deaths) have been reported in clinical efficacy studies in patients with elevated cyclosporine or tacrolimus concentrations. Frequent monitoring of tacrolimus or cyclosporine whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the tacrolimus or cyclosporine dose adjusted accordingly.
5.2 Arrhythmias and QT Prolongation
Some azoles, including posaconazole, have been associated with prolongation of the QT interval on the electrocardiogram. In addition, cases of torsades de pointes have been reported in patients taking posaconazole.
Results from a multiple time-matched ECG analysis in healthy volunteers did not show any increase in the mean of the QTc interval. Multiple, time-matched ECGs collected over a 12-hour period were recorded at baseline and steady-state from 173 healthy male and female volunteers (18-85 years of age) administered posaconazole oral suspension 400 mg BID with a high-fat meal. In this pooled analysis, the mean QTc (Fridericia) interval change from baseline was –5 msec following administration of the recommended clinical dose. A decrease in the QTc(F) interval (–3 msec) was also observed in a small number of subjects (n=16) administered placebo. The placebo-adjusted mean maximum QTc(F) interval change from baseline was <0 msec (–8 msec). No healthy subject administered posaconazole had a QTc(F) interval ≥500 msec or an increase ≥60 msec in their QTc(F) interval from baseline.
Posaconazole should be administered with caution to patients with potentially proarrhythmic conditions. Do not administer with drugs that are known to prolong the QTc interval and are metabolized through CYP3A4 [see Contraindications (4.3) and Drug Interactions (7.2)].
5.3 Electrolyte Disturbances
Electrolyte disturbances, especially those involving potassium, magnesium or calcium levels, should be monitored and corrected as necessary before and during posaconazole therapy.
5.4 Hepatic Toxicity
Hepatic reactions (e.g., mild to moderate elevations in alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, total bilirubin, and/or clinical hepatitis) have been reported in clinical trials. The elevations in liver function tests were generally reversible on discontinuation of therapy, and in some instances these tests normalized without drug interruption. Cases of more severe hepatic reactions including cholestasis or hepatic failure including deaths have been reported in patients with serious underlying medical conditions (e.g., hematologic malignancy) during treatment with posaconazole. These severe hepatic reactions were seen primarily in subjects receiving the posaconazole oral suspension 800 mg daily (400 mg BID or 200 mg QID) in clinical trials.
Liver function tests should be evaluated at the start of and during the course of posaconazole therapy. Patients who develop abnormal liver function tests during posaconazole therapy should be monitored for the development of more severe hepatic injury. Patient management should include laboratory evaluation of hepatic function (particularly liver function tests and bilirubin). Discontinuation of posaconazole must be considered if clinical signs and symptoms consistent with liver disease develop that may be attributable to posaconazole.
5.5 Renal Impairment
Due to the variability in exposure with posaconazole delayed-release tablets and oral suspension, patients with severe renal impairment should be monitored closely for breakthrough fungal infections [see Dosage and Administration (2.6) and Use in Specific Populations (8.6)].
5.6 Use with Midazolam
Concomitant administration of posaconazole with midazolam increases the midazolam plasma concentrations by approximately 5-fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Patients must be monitored closely for adverse effects associated with high plasma concentrations of midazolam and benzodiazepine receptor antagonists must be available to reverse these effects [see Drug Interactions (7.5) and Clinical Pharmacology (12.3)].
5.7 Vincristine Toxicity
Concomitant administration of azole antifungals, including posaconazole, with vincristine has been associated with neurotoxicity and other serious adverse reactions, including seizures, peripheral neuropathy, syndrome of inappropriate antidiuretic hormone secretion, and paralytic ileus. Reserve azole antifungals, including posaconazole, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options [see Drug Interactions (7.10)].
-
6 ADVERSE REACTIONS
The following serious and otherwise important adverse reactions are discussed in detail in another section of the labeling:
- Hypersensitivity [see Contraindications (4.1)]
- Arrhythmias and QT Prolongation [see Warnings and Precautions (5.2)]
- Hepatic Toxicity [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of posaconazole cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. In clinical trials, the type of adverse reactions reported for posaconazole delayed-release tablets were generally similar to that reported in trials of posaconazole oral suspension.
Clinical Trial Experience with Posaconazole Delayed-Release Tablets
The safety of posaconazole delayed-release tablets has been assessed in 230 patients in clinical trials. Patients were enrolled in a non-comparative pharmacokinetic and safety trial of posaconazole delayed-release tablets when given as antifungal prophylaxis (Delayed-Release Tablet Study 1). Patients were immunocompromised with underlying conditions including hematological malignancy, neutropenia post-chemotherapy, GVHD, and post HSCT. This patient population was 62% male, had a mean age of 51 years (range 19-78 years, 17% of patients were ≥65 years of age), and were 93% white and 16% Hispanic. Posaconazole therapy was given for a median duration of 28 days. Twenty patients received 200 mg daily dose and 210 patients received 300 mg daily dose (following twice daily dosing on Day 1 in each cohort). Table 2 presents treatment-emergent adverse reactions observed in patients treated with 300 mg daily dose at an incidence of ≥10% in posaconazole delayed-release tablet study.
Table 2: Posaconazole Delayed-Release Tablet Study 1: Number (%) of Subjects Treated with 300 mg Daily Dose Reporting Treatment-Emergent Adverse Reactions: Frequency of at Least 10% Body System
Preferred TermPosaconazole delayed-release tablet (300 mg)
(n=210)Subjects Reporting any Adverse Reaction 201 (99) Blood and Lymphatic System Disorder Anemia 22 (10) Thrombocytopenia 29 (14) Gastrointestinal Disorders Abdominal Pain 23 (11) Constipation 20 (10) Diarrhea 61 (29) Nausea 56 (27) Vomiting 28 (13) General Disorders and Administration Site Conditions Asthenia 20 (10) Chills 22 (10) Mucosal Inflammation 29 (14) Edema Peripheral 33 (16) Pyrexia 59 (28) Metabolism and Nutrition Disorders Hypokalemia 46 (22) Hypomagnesemia 20 (10) Nervous System Disorders Headache 30 (14) Respiratory, Thoracic and Mediastinal Disorders Cough 35 (17) Epistaxis 30 (14) Skin and Subcutaneous Tissue Disorders Rash 34 (16) Vascular Disorders Hypertension 23 (11) The most frequently reported adverse reactions (>25%) with posaconazole delayed-release tablets 300 mg once daily were diarrhea, pyrexia, and nausea.
The most common adverse reaction leading to discontinuation of posaconazole delayed-release tablets 300 mg once daily was nausea (2%).
Clinical Trial Safety Experience with Posaconazole Oral Suspension
The safety of posaconazole oral suspension has been assessed in 1844 patients. This includes 605 patients in the active-controlled prophylaxis studies, 557 patients in the active-controlled OPC studies, 239 patients in refractory OPC studies, and 443 patients from other indications. This represents a heterogeneous population, including immunocompromised patients, e.g., patients with hematological malignancy, neutropenia post-chemotherapy, GVHD post HSCT, and HIV infection, as well as non-neutropenic patients. This patient population was 71% male, had a mean age of 42 years (range 8-84 years, 6% of patients were ≥65 years of age and 1% was <18 years of age), and were 64% white, 16% Hispanic, and 36% non-white (including 14% black). Posaconazole therapy was given to 171 patients for ≥6 months, with 58 patients receiving posaconazole therapy for ≥12 months. Table 3 presents treatment-emergent adverse reactions observed at an incidence of >10% in posaconazole prophylaxis studies. Table 4 presents treatment-emergent adverse reactions observed at an incidence of at least 10% in the OPC/rOPC studies.
Prophylaxis of Aspergillus and Candida: In the 2 randomized, comparative prophylaxis studies (Oral Suspension Studies 1 and 2), the safety of posaconazole oral suspension 200 mg three times a day was compared to fluconazole 400 mg once daily or itraconazole 200 mg twice a day in severely immunocompromised patients.
The most frequently reported adverse reactions (>30%) in the prophylaxis clinical trials were fever, diarrhea, and nausea.
The most common adverse reactions leading to discontinuation of posaconazole in the prophylaxis studies were associated with GI disorders, specifically, nausea (2%), vomiting (2%), and hepatic enzymes increased (2%).
Table 3: Posaconazole Oral Suspension Study 1 and Study 2. Number (%) of Randomized Subjects Reporting Treatment-Emergent Adverse Reactions: Frequency of at Least 10% in the Posaconazole Oral Suspension or Fluconazole Treatment Groups (Pooled Prophylaxis Safety Analysis) Body System
Preferred TermPosaconazole
(n=605)Fluconazole
(n=539)Itraconazole
(n=58)- * Percentages of sex-specific adverse reactions are based on the number of males/females.
Subjects Reporting any Adverse Reaction 595 (98) 531 (99) 58 (100) Body as a Whole - General Disorders Fever 274 (45) 254 (47) 32 (55) Headache 171 (28) 141 (26) 23 (40) Rigors 122 (20) 87 (16) 17 (29) Fatigue 101 (17) 98 (18) 5 (9) Edema Legs 93 (15) 67 (12) 11 (19) Anorexia 92 (15) 94 (17) 16 (28) Dizziness 64 (11) 56 (10) 5 (9) Edema 54 (9) 68 (13) 8 (14) Weakness 51 (8) 52 (10) 2 (3) Cardiovascular Disorders, General Hypertension 106 (18) 88 (16) 3 (5) Hypotension 83 (14) 79 (15) 10 (17) Disorders of Blood and Lymphatic System Anemia 149 (25) 124 (23) 16 (28) Neutropenia 141 (23) 122 (23) 23 (40) Disorders of the Reproductive System and Breast Vaginal Hemorrhage* 24 (10) 20 (9) 3 (12) Gastrointestinal System Disorders Diarrhea 256 (42) 212 (39) 35 (60) Nausea 232 (38) 198 (37) 30 (52) Vomiting 174 (29) 173 (32) 24 (41) Abdominal Pain 161 (27) 147 (27) 21 (36) Constipation 126 (21) 94 (17) 10 (17) Dyspepsia 61 (10) 50 (9) 6 (10) Heart Rate and Rhythm Disorders Tachycardia 72 (12) 75 (14) 3 (5) Infection and Infestations Pharyngitis 71 (12) 60 (11) 12 (21) Liver and Biliary System Disorders Bilirubinemia 59 (10) 51 (9) 11 (19) Metabolic and Nutritional Disorders Hypokalemia 181 (30) 142 (26) 30 (52) Hypomagnesemia 110 (18) 84 (16) 11 (19) Hyperglycemia 68 (11) 76 (14) 2 (3) Hypocalcemia 56 (9) 55 (10) 5 (9) Musculoskeletal System Disorders Musculoskeletal Pain 95 (16) 82 (15) 9 (16) Arthralgia 69 (11) 67 (12) 5 (9) Back Pain 63 (10) 66 (12) 4 (7) Platelet, Bleeding and Clotting Disorders Thrombocytopenia 175 (29) 146 (27) 20 (34) Petechiae 64 (11) 54 (10) 9 (16) Psychiatric Disorders Insomnia 103 (17) 92 (17) 11 (19) Respiratory System Disorders Coughing 146 (24) 130 (24) 14 (24) Dyspnea 121 (20) 116 (22) 15 (26) Epistaxis 82 (14) 73 (14) 12 (21) Skin and Subcutaneous Tissue Disorders Rash 113 (19) 96 (18) 25 (43) Pruritus 69 (11) 62 (12) 11 (19) HIV Infected Subjects with OPC: In 2 randomized comparative studies in OPC, the safety of posaconazole oral suspension at a dose of less than or equal to 400 mg QD in 557 HIV-infected patients was compared to the safety of fluconazole in 262 HIV-infected patients at a dose of 100 mg QD.
An additional 239 HIV-infected patients with refractory OPC received posaconazole oral suspension in 2 non-comparative trials for refractory OPC (rOPC). Of these subjects, 149 received the 800-mg/day dose and the remainder received the less than or equal to 400-mg QD dose.
In the OPC/rOPC studies, the most common adverse reactions were fever, diarrhea, nausea, headache, vomiting, and coughing.
The most common adverse reactions that led to treatment discontinuation of posaconazole in the Controlled OPC Pool included respiratory impairment (1%) and pneumonia (1%). In the refractory OPC pool, the most common adverse reactions that led to treatment discontinuation of posaconazole were AIDS (7%) and respiratory impairment (3%).
Table 4: Treatment-Emergent Adverse Reactions with Frequency of at Least 10% in OPC Studies with Posaconazole Oral Suspension (Treated Population) Body System
Preferred TermNumber (%) of Subjects Controlled OPC Pool Refractory OPC Pool Posaconazole Fluconazole Posaconazole n=557 n=262 n=239 OPC=oropharyngeal candidiasis - * Number of subjects reporting treatment-emergent adverse reactions at least once during the study, without regard to relationship to treatment. Subjects may have reported more than 1 event.
Subjects Reporting any Adverse Reaction* 356 (64) 175 (67) 221 (92) Body as a Whole – General Disorders Fever 34 (6) 22 (8) 82 (34) Headache 44 (8) 23 (9) 47 (20) Anorexia 10 (2) 4 (2) 46 (19) Fatigue 18 (3) 12 (5) 31 (13) Asthenia 9 (2) 5 (2) 31 (13) Rigors 2 (<1) 4 (2) 29 (12) Pain 4 (1) 2 (1) 27 (11) Disorders of Blood and Lymphatic System Neutropenia 21 (4) 8 (3) 39 (16) Anemia 11 (2) 5 (2) 34 (14) Gastrointestinal System Disorders Diarrhea 58 (10) 34 (13) 70 (29) Nausea 48 (9) 30 (11) 70 (29) Vomiting 37 (7) 18 (7) 67 (28) Abdominal Pain 27 (5) 17 (6) 43 (18) Infection and Infestations Candidiasis, Oral 3 (1) 1 (<1) 28 (12) Herpes Simplex 16 (3) 8 (3) 26 (11) Pneumonia 17 (3) 6 (2) 25 (10) Metabolic and Nutritional Disorders Weight Decrease 4 (1) 2 (1) 33 (14) Dehydration 4 (1) 7 (3) 27 (11) Psychiatric Disorders Insomnia 8 (1) 3 (1) 39 (16) Respiratory System Disorders Coughing 18 (3) 11 (4) 60 (25) Dyspnea 8 (1) 8 (3) 28 (12) Skin and Subcutaneous Tissue Disorders Rash 15 (3) 10 (4) 36 (15) Sweating Increased 13 (2) 5 (2) 23 (10) Adverse reactions were reported more frequently in the pool of patients with refractory OPC. Among these highly immunocompromised patients with advanced HIV disease, serious adverse reactions (SARs) were reported in 55% (132/239). The most commonly reported SARs were fever (13%) and neutropenia (10%).
Less Common Adverse Reactions: Clinically significant adverse reactions reported during clinical trials in prophylaxis, OPC/rOPC or other trials with posaconazole which occurred in less than 5% of patients are listed below:
- Blood and lymphatic system disorders: hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, neutropenia aggravated
- Endocrine disorders: adrenal insufficiency
- Nervous system disorders: paresthesia
- Immune system disorders: allergic reaction [see Contraindications (4.1)]
- Cardiac disorders: torsades de pointes [see Warnings and Precautions (5.2)]
- Vascular disorders: pulmonary embolism
- Gastrointestinal disorders: pancreatitis
- Liver and Biliary System Disorders: bilirubinemia, hepatic enzymes increased, hepatic function abnormal, hepatitis, hepatomegaly, jaundice, AST Increased, ALT Increased
- Metabolic and Nutritional Disorders: hypokalemia
- Platelet, Bleeding, and Clotting Disorders: thrombocytopenia
- Renal & Urinary System Disorders: renal failure acute
Clinical Laboratory Values: In healthy volunteers and patients, elevation of liver function test values did not appear to be associated with higher plasma concentrations of posaconazole.
For the prophylaxis studies, the number of patients with changes in liver function tests from Common Toxicity Criteria (CTC) Grade 0, 1, or 2 at baseline to Grade 3 or 4 during the study is presented in Table 5.
Table 5: Posaconazole Oral Suspension Study 1 and Study 2. Changes in Liver Function Test Results from CTC Grade 0, 1, or 2 at Baseline to Grade 3 or 4 Number (%) of Patients with Change* CTC = Common Toxicity Criteria; AST= Aspartate Aminotransferase; ALT= Alanine Aminotransferase. - * Change from Grade 0 to 2 at baseline to Grade 3 or 4 during the study. These data are presented in the form X/Y, where X represents the number of patients who met the criterion as indicated, and Y represents the number of patients who had a baseline observation and at least one post-baseline observation.
Oral Suspension Study 1 Laboratory Parameter Posaconazole
n=301Fluconazole
n=299AST 11/266 (4) 13/266 (5) ALT 47/271 (17) 39/272 (14) Bilirubin 24/271 (9) 20/275 (7) Alkaline Phosphatase 9/271 (3) 8/271 (3) Oral Suspension Study 2 Laboratory Parameter Posaconazole
(n=304)Fluconazole/Itraconazole
(n=298)AST 9/286 (3) 5/280 (2) ALT 18/289 (6) 13/284 (5) Bilirubin 20/290 (7) 25/285 (9) Alkaline Phosphatase 4/281 (1) 1/276 (<1) The number of patients treated for OPC with clinically significant liver function test (LFT) abnormalities at any time during the studies is provided in Table 6 (LFT abnormalities were present in some of these patients prior to initiation of the study drug).
Table 6: Posaconazole Oral Suspension Studies: Clinically Significant Laboratory Test Abnormalities without Regard to Baseline Value Laboratory Test Controlled Refractory Posaconazole Fluconazole Posaconazole n=557(%) n=262(%) n=239(%) ALT= Alanine Aminotransferase; AST= Aspartate Aminotransferase. ALT > 3.0 × ULN 16/537 (3) 13/254 (5) 25/226 (11) AST > 3.0 × ULN 33/537 (6) 26/254 (10) 39/223 (17) Total Bilirubin > 1.5 × ULN 15/536 (3) 5/254 (2) 9/197 (5) Alkaline Phosphatase > 3.0 × ULN 17/535 (3) 15/253 (6) 24/190 (13) 6.2 Postmarketing Experience
The following adverse reaction has been identified during the post-approval use of posaconazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency.
Endocrine Disorders: Pseudoaldosteronism
-
7 DRUG INTERACTIONS
Posaconazole is primarily metabolized via UDP glucuronosyltransferase and is a substrate of p-glycoprotein (P-gp) efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. Coadministration of drugs that can decrease the plasma concentrations of posaconazole should generally be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
Posaconazole is also a strong inhibitor of CYP3A4. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole [see Clinical Pharmacology (12.3)].
The following information was derived from data with posaconazole oral suspension or early tablet formulation. All drug interactions with posaconazole oral suspension, except for those that affect the absorption of posaconazole (via gastric pH and motility) are considered relevant to posaconazole injection as well [see Drug Interactions (7.9) and (7.13)].
7.1 Immunosuppressants Metabolized by CYP3A4
Sirolimus: Concomitant administration of posaconazole with sirolimus increases the sirolimus blood concentrations by approximately 9-fold and can result in sirolimus toxicity. Therefore, posaconazole is contraindicated with sirolimus [see Contraindications (4.2) and Clinical Pharmacology (12.3)].
Tacrolimus: Posaconazole has been shown to significantly increase the Cmax and AUC of tacrolimus. At initiation of posaconazole treatment, reduce the tacrolimus dose to approximately one-third of the original dose. Frequent monitoring of tacrolimus whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the tacrolimus dose adjusted accordingly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
Cyclosporine: Posaconazole has been shown to increase cyclosporine whole blood concentrations in heart transplant patients upon initiation of posaconazole treatment. It is recommended to reduce cyclosporine dose to approximately three-fourths of the original dose upon initiation of posaconazole treatment. Frequent monitoring of cyclosporine whole blood trough concentrations should be performed during and at discontinuation of posaconazole treatment and the cyclosporine dose adjusted accordingly [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.2 CYP3A4 Substrates
Concomitant administration of posaconazole with CYP3A4 substrates such as pimozide and quinidine may result in increased plasma concentrations of these drugs, leading to QTc prolongation and cases of torsades de pointes. Therefore, posaconazole is contraindicated with these drugs [see Contraindications (4.3) and Warnings and Precautions (5.2)].
7.3 HMG-CoA Reductase Inhibitors (Statins) Primarily Metabolized Through CYP3A4
Concomitant administration of posaconazole with simvastatin increases the simvastatin plasma concentrations by approximately 10-fold. Therefore, posaconazole is contraindicated with HMG-CoA reductase inhibitors primarily metabolized through CYP3A4 [see Contraindications (4.4) and Clinical Pharmacology (12.3)].
7.4 Ergot Alkaloids
Most of the ergot alkaloids are substrates of CYP3A4. Posaconazole may increase the plasma concentrations of ergot alkaloids (ergotamine and dihydroergotamine) which may lead to ergotism. Therefore, posaconazole is contraindicated with ergot alkaloids [see Contraindications (4.5)].
7.5 Benzodiazepines Metabolized by CYP3A4
Concomitant administration of posaconazole with midazolam increases the midazolam plasma concentrations by approximately 5-fold. Increased plasma midazolam concentrations could potentiate and prolong hypnotic and sedative effects. Concomitant use of posaconazole and other benzodiazepines metabolized by CYP3A4 (e.g., alprazolam, triazolam) could result in increased plasma concentrations of these benzodiazepines. Patients must be monitored closely for adverse effects associated with high plasma concentrations of benzodiazepines metabolized by CYP3A4 and benzodiazepine receptor antagonists must be available to reverse these effects [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
7.6 Anti-HIV Drugs
Efavirenz: Efavirenz induces UDP-glucuronidase and significantly decreases posaconazole plasma concentrations [see Clinical Pharmacology (12.3)]. It is recommended to avoid concomitant use of efavirenz with posaconazole unless the benefit outweighs the risks.
Ritonavir and Atazanavir: Ritonavir and atazanavir are metabolized by CYP3A4 and posaconazole increases plasma concentrations of these drugs [see Clinical Pharmacology (12.3)]. Frequent monitoring of adverse effects and toxicity of ritonavir and atazanavir should be performed during coadministration with posaconazole.
Fosamprenavir: Combining fosamprenavir with posaconazole may lead to decreased posaconazole plasma concentrations. If concomitant administration is required, close monitoring for breakthrough fungal infections is recommended [see Clinical Pharmacology (12.3)].
7.7 Rifabutin
Rifabutin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Rifabutin is also metabolized by CYP3A4. Therefore, coadministration of rifabutin with posaconazole increases rifabutin plasma concentrations [see Clinical Pharmacology (12.3)]. Concomitant use of posaconazole and rifabutin should be avoided unless the benefit to the patient outweighs the risk. However, if concomitant administration is required, close monitoring for breakthrough fungal infections as well as frequent monitoring of full blood counts and adverse reactions due to increased rifabutin plasma concentrations (e.g., uveitis, leukopenia) are recommended.
7.8 Phenytoin
Phenytoin induces UDP-glucuronidase and decreases posaconazole plasma concentrations. Phenytoin is also metabolized by CYP3A4. Therefore, coadministration of phenytoin with posaconazole increases phenytoin plasma concentrations [see Clinical Pharmacology (12.3)]. Concomitant use of posaconazole and phenytoin should be avoided unless the benefit to the patient outweighs the risk. However, if concomitant administration is required, close monitoring for breakthrough fungal infections is recommended and frequent monitoring of phenytoin concentrations should be performed while coadministered with posaconazole and dose reduction of phenytoin should be considered.
7.9 Gastric Acid Suppressors/Neutralizers
Posaconazole Delayed-Release Tablet:
No clinically relevant effects on the pharmacokinetics of posaconazole were observed when posaconazole delayed-release tablets are concomitantly used with antacids, H2-receptor antagonists and proton pump inhibitors [see Clinical Pharmacology (12.3)]. No dosage adjustment of posaconazole delayed-release tablets is required when posaconazole delayed-release tablets are concomitantly used with antacids, H2-receptor antagonists and proton pump inhibitors.
7.10 Vinca Alkaloids
Most of the vinca alkaloids (e.g., vincristine and vinblastine) are substrates of CYP3A4. Concomitant administration of azole antifungals, including posaconazole, with vincristine has been associated with serious adverse reactions [see Warnings and Precautions (5.7)]. Posaconazole may increase the plasma concentrations of vinca alkaloids which may lead to neurotoxicity and other serious adverse reactions. Therefore, reserve azole antifungals, including posaconazole, for patients receiving a vinca alkaloid, including vincristine, who have no alternative antifungal treatment options.
7.11 Calcium Channel Blockers Metabolized by CYP3A4
Posaconazole may increase the plasma concentrations of calcium channel blockers metabolized by CYP3A4 (e.g., verapamil, diltiazem, nifedipine, nicardipine, felodipine). Frequent monitoring for adverse reactions and toxicity related to calcium channel blockers is recommended during coadministration. Dose reduction of calcium channel blockers may be needed.
7.12 Digoxin
Increased plasma concentrations of digoxin have been reported in patients receiving digoxin and posaconazole. Therefore, monitoring of digoxin plasma concentrations is recommended during coadministration.
7.13 Gastrointestinal Motility Agents
Posaconazole Delayed-Release Tablet:
Concomitant administration of metoclopramide with posaconazole delayed-release tablets did not affect the pharmacokinetics of posaconazole [see Clinical Pharmacology (12.3)]. No dosage adjustment of posaconazole delayed-release tablets is required when given concomitantly with metoclopramide.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal data, posaconazole may cause fetal harm when administered to pregnant women. Available data for use of posaconazole in pregnant women are insufficient to establish a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, skeletal malformations (cranial malformations and missing ribs) and maternal toxicity (reduced food consumption and reduced body weight gain) were observed when posaconazole was dosed orally to pregnant rats during organogenesis at doses ≥1.4 times the 400 mg twice daily oral suspension regimen based on steady-state plasma concentrations of posaconazole in healthy volunteers. In pregnant rabbits dosed orally during organogenesis, increased resorptions, reduced litter size, and reduced body weight gain of females were seen at doses 5 times the exposure achieved with the 400 mg twice daily oral suspension regimen. Doses of ≥ 3 times the clinical exposure caused an increase in resorptions in these rabbits (see Data). Based on animal data, advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Posaconazole resulted in maternal toxicity (reduced food consumption and reduced body weight gain) and skeletal malformations (cranial malformations and missing ribs) when given orally to pregnant rats during organogenesis (Gestational Days 6 through 15) at doses ≥27 mg/kg (≥1.4 times the 400 mg twice daily oral suspension regimen based on steady-state plasma concentrations of drug in healthy volunteers). The no-effect dose for malformations and maternal toxicity in rats was 9 mg/kg, which is 0.7 times the exposure achieved with the 400 mg twice daily oral suspension regimen. No malformations were seen in rabbits dosed during organogenesis (Gestational Days 7 through 19) at doses up to 80 mg/kg (5 times the exposure achieved with the 400 mg twice daily oral suspension regimen). In the rabbit, the no-effect dose was 20 mg/kg, while high doses of 40 mg/kg and 80 mg/kg (3 or 5 times the clinical exposure) caused an increase in resorptions. In rabbits dosed at 80 mg/kg, a reduction in body weight gain of females and a reduction in litter size were seen.
8.2 Lactation
Risk Summary
There are no data on the presence of posaconazole in human milk, the effects on the breastfed infant, or the effects on milk production. Posaconazole is excreted in the milk of lactating rats. When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for posaconazole and any potential adverse effects on the breastfed child from posaconazole or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of posaconazole oral suspension and posaconazole delayed-release tablets have been established in the age groups 13 to 17 years of age. Use of posaconazole in these age groups is supported by evidence from adequate and well-controlled studies of posaconazole in adults. The safety and effectiveness of posaconazole in pediatric patients below the age of 13 years (birth to 12 years) have not been established.
A total of 12 patients 13 to 17 years of age received 600 mg/day (200 mg three times a day) of posaconazole oral suspension for prophylaxis of invasive fungal infections. The safety profile in these patients <18 years of age appears similar to the safety profile observed in adults. Based on pharmacokinetic data in 10 of these pediatric patients, the mean steady-state average posaconazole concentration (Cavg) was similar between these patients and adults (≥18 years of age). In a study of 136 neutropenic pediatric patients 11 months to less than 18 years treated with posaconazole oral suspension, the exposure target of steady-state posaconazole Cavg between 500 ng/mL and less than 2500 ng/mL was attained in approximately 50% of patients instead of the pre-specified 90% of patients.
8.5 Geriatric Use
Of the 230 patients treated with posaconazole delayed-release tablets, 38 (17%) were greater than 65 years of age. The pharmacokinetics of posaconazole delayed-release tablets are comparable in young and elderly subjects. No overall differences in safety were observed between the geriatric patients and younger patients; therefore, no dosage adjustment is recommended for geriatric patients.
Of the 605 patients randomized to posaconazole oral suspension in the prophylaxis clinical trials, 63 (10%) were ≥65 years of age. In addition, 48 patients treated with greater than or equal to 800-mg/day posaconazole in another indication were ≥65 years of age. No overall differences in safety were observed between the geriatric patients and younger patients.
No overall differences in the pharmacokinetics and safety were observed between elderly and young subjects during clinical trials, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
Following single-dose administration of 400 mg of the oral suspension, there was no significant effect of mild (eGFR: 50-80 mL/min/1.73 m2, n=6) or moderate (eGFR: 20-49 mL/min/1.73 m2, n=6) renal impairment on posaconazole pharmacokinetics; therefore, no dose adjustment is required in patients with mild to moderate renal impairment. In subjects with severe renal impairment (eGFR: <20 mL/min/1.73 m2), the mean plasma exposure (AUC) was similar to that in patients with normal renal function (eGFR: >80 mL/min/1.73 m2); however, the range of the AUC estimates was highly variable (CV=96%) in these subjects with severe renal impairment as compared to that in the other renal impairment groups (CV<40%). Due to the variability in exposure, patients with severe renal impairment should be monitored closely for breakthrough fungal infections [see Dosage and Administration (2)]. Similar recommendations apply to posaconazole delayed-release tablets; however, a specific study has not been conducted with the delayed-release tablets.
8.7 Hepatic Impairment
After a single oral dose of posaconazole oral suspension 400 mg, the mean AUC was 43%, 27%, and 21% higher in subjects with mild (Child-Pugh Class A, N=6), moderate (Child-Pugh Class B, N=6), or severe (Child-Pugh Class C, N=6) hepatic impairment, respectively, compared to subjects with normal hepatic function (N=18). Compared to subjects with normal hepatic function, the mean Cmax was 1% higher, 40% higher, and 34% lower in subjects with mild, moderate, or severe hepatic impairment, respectively. The mean apparent oral clearance (CL/F) was reduced by 18%, 36%, and 28% in subjects with mild, moderate, or severe hepatic impairment, respectively, compared to subjects with normal hepatic function. The elimination half-life (t½) was 27 hours, 39 hours, 27 hours, and 43 hours in subjects with normal hepatic function and mild, moderate, or severe hepatic impairment, respectively.
It is recommended that no dose adjustment of posaconazole is needed in patients with mild to severe hepatic impairment (Child-Pugh Class A, B, or C) [see Dosage and Administration (2) and Warnings and Precautions (5.4)]. Similar recommendations apply to posaconazole delayed-release tablets; however, a specific study has not been conducted with the delayed-release tablets.
8.8 Gender
The pharmacokinetics of posaconazole are comparable in men and women. No adjustment in the dosage of posaconazole is necessary based on gender.
-
10 OVERDOSAGE
There is no experience with overdosage of posaconazole delayed-release tablets.
During the clinical trials, some patients received posaconazole oral suspension up to 1600 mg/day with no adverse reactions noted that were different from the lower doses. In addition, accidental overdose was noted in one patient who took 1200 mg BID posaconazole oral suspension for 3 days. No related adverse reactions were noted by the investigator.
Posaconazole is not removed by hemodialysis.
-
11 DESCRIPTION
Posaconazole is an azole antifungal agent available as delayed-release tablet or suspension for oral administration.
Posaconazole is designated chemically as 4-[4-[4-[4-[[ (3R,5R)-5- (2,4-difluorophenyl)tetrahydro-5- (1H-1,2,4-triazol-1-ylmethyl)-3-furanyl]methoxy]phenyl]-1-piperazinyl]phenyl]-2-[(1S,2S)-1-ethyl-2-hydroxypropyl]-2,4-dihydro-3H-1,2,4-triazol-3-one with an empirical formula of C37H42F2N8O4 and a molecular weight of 700.8. The chemical structure is:
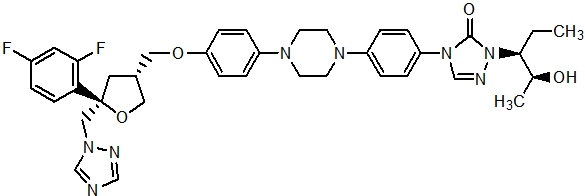
Posaconazole is a white powder with a low aqueous solubility.
Posaconazole delayed-release tablet is a yellow, coated, oblong tablet containing 100 mg of posaconazole. Each delayed-release tablet contains the inactive ingredients: hypromellose acetate succinate, microcrystalline cellulose, hydroxypropylcellulose, silicon dioxide, croscarmellose sodium, magnesium stearate, and Opadry® II Yellow (consists of the following ingredients: polyvinyl alcohol partially hydrolyzed, Macrogol/PEG 3350, titanium dioxide, talc, and iron oxide yellow).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Posaconazole is an azole antifungal agent [see Clinical Pharmacology (12.4)].
12.2 Pharmacodynamics
Exposure Response Relationship: In clinical studies of neutropenic patients who were receiving cytotoxic chemotherapy for acute myelogenous leukemia (AML) or myelodysplastic syndromes (MDS) or hematopoietic stem cell transplant (HSCT) recipients with Graft versus Host Disease (GVHD), a wide range of plasma exposures to posaconazole was noted following administration of posaconazole oral suspension. A pharmacokinetic-pharmacodynamic analysis of patient data revealed an apparent association between average posaconazole concentrations (Cavg) and prophylactic efficacy (Table 7). A lower Cavg may be associated with an increased risk of treatment failure, defined as treatment discontinuation, use of empiric systemic antifungal therapy (SAF), or occurrence of breakthrough invasive fungal infections.
Table 7: Posaconazole Oral Suspension Exposure Analysis (Cavg) in Prophylaxis Trials Prophylaxis in AML/MDS* Prophylaxis in GVHD† Cavg Range (ng/mL) Treatment Failure‡ (%) Cavg Range (ng/mL) Treatment Failure‡ (%) Cavg = the average posaconazole concentration when measured at steady state - * Neutropenic patients who were receiving cytotoxic chemotherapy for AML or MDS
- † HSCT recipients with GVHD
- ‡ Defined as treatment discontinuation, use of empiric systemic antifungal therapy (SAF), or occurrence of breakthrough invasive fungal infections
Quartile 1 90-322 54.7 22-557 44.4 Quartile 2 322-490 37.0 557-915 20.6 Quartile 3 490-734 46.8 915-1563 17.5 Quartile 4 734-2200 27.8 1563-3650 17.5 12.3 Pharmacokinetics
General Pharmacokinetic Characteristics
Posaconazole Delayed-Release Tablets
Posaconazole delayed-release tablets exhibit dose proportional pharmacokinetics after single and multiple dosing up to 300 mg. The mean pharmacokinetic parameters of posaconazole at steady state following administration of posaconazole delayed-release tablets 300 mg twice daily (BID) on Day 1, then 300 mg once daily (QD) thereafter in healthy volunteers and in neutropenic patients who are receiving cytotoxic chemotherapy for AML or MDS or HSCT recipients with GVHD are shown in Table 8.
Table 8: Arithmetic Mean (%CV) of Steady State PK Parameters in Healthy Volunteers and Patients Following Administration of Posaconazole Delayed-Release Tablets (300 mg)* N AUC0-24 hr
(ng∙hr/mL)Cav†
(ng/mL)Cmax
(ng/mL)Cmin
(ng/mL)Tmax‡
(hr)t1/2
(hr)CL/F
(L/hr)CV = coefficient of variation expressed as a percentage (%CV); AUC0-T = Area under the plasma concentration-time curve from time zero to 24 hr; Cmax = maximum observed concentration; Cmin = minimum observed plasma concentration; Tmax = time of maximum observed concentration; t½ = terminal phase half-life; CL /F = Apparent total body clearance - * 300 mg BID on Day 1, then 300 mg QD thereafter
- † Cav = time-averaged concentrations (i.e., AUC0-24 hr/24hr)
- ‡ Median (minimum-maximum)
Healthy Volunteers 12 51618
(25)2151
(25)2764
(21)1785
(29)4
(3-6)31
(40)7.5
(26)Patients 50 37900
(42)1580
(42)2090
(38)1310
(50)4 (1.3-8.3) - 9.39
(45)Posaconazole Oral Suspension
Dose-proportional increases in plasma exposure (AUC) to posaconazole oral suspension were observed following single oral doses from 50 mg to 800 mg and following multiple-dose administration from 50 mg BID to 400 mg BID in healthy volunteers. No further increases in exposure were observed when the dose of the oral suspension increased from 400 mg BID to 600 mg BID in febrile neutropenic patients or those with refractory invasive fungal infections.
The mean (%CV) [min-max] posaconazole oral suspension average steady-state plasma concentrations (Cavg) and steady-state pharmacokinetic parameters in patients following administration of 200 mg TID and 400 mg BID of the oral suspension are provided in Table 9.
Table 9: The Mean (%CV) [min-max] Posaconazole Steady-State Pharmacokinetic Parameters in Patients Following Oral Administration of Posaconazole Oral Suspension 200 mg TID and 400 mg BID Dose* Cavg (ng/mL) AUC† (ng∙hr/mL) CL/F (L/hr) V/F (L) t½ (hr) Cavg = the average posaconazole concentration when measured at steady state - * Oral suspension administration
- † AUC (0-24 hr) for 200 mg TID and AUC (0-12 hr) for 400 mg BID
- ‡ HSCT recipients with GVHD
- § Not done
- ¶ Neutropenic patients who were receiving cytotoxic chemotherapy for acute myelogenous leukemia or myelodysplastic syndromes
- # Febrile neutropenic patients or patients with refractory invasive fungal infections, Cavg n=24
The variability in average plasma posaconazole concentrations in patients was relatively higher than that in healthy subjects.200 mg TID‡ (n=252) 1103 (67)
[21.5-3650]ND§ ND§ ND§ ND§ 200 mg TID¶ (n=215) 583 (65)
[89.7-2200]15,900 (62)
[4100-56,100]51.2 (54)
[10.7-146]2425 (39)
[828-5702]37.2 (39)
[19.1-148]400 mg BID# (n=23) 723 (86)
[6.70-2256]9093 (80)
[1564-26,794]76.1 (78)
[14.9-256]3088 (84)
[407-13,140]31.7 (42)
[12.4-67.3]Absorption:
Posaconazole Delayed-Release Tablets
When given orally in healthy volunteers, posaconazole delayed-release tablets are absorbed with a median Tmax of 4 to 5 hours. Steady-state plasma concentrations are attained by Day 6 at the 300 mg dose (QD after BID loading dose at Day 1). The absolute bioavailability of the oral delayed-release tablet is approximately 54% under fasted conditions. The Cmax and AUC of posaconazole following administration of posaconazole delayed-release tablets is increased 16% and 51%, respectively, when given with a high fat meal compared to a fasted state (see Table 10). In order to enhance the oral absorption of posaconazole and optimize plasma concentrations, posaconazole delayed-release tablets should be administered with food.
Table 10: Statistical Comparison of Plasma Pharmacokinetics of Posaconazole Following Single Oral Dose Administration of 300 mg Posaconazole Delayed-Release Tablet to Healthy Subjects under Fasting and Fed Conditions Fasting Conditions Fed Conditions
(High Fat Meal)*Fed/Fasting Pharmacokinetic Parameter N Mean (%CV) N Mean (%CV) GMR (90% CI) GMR=Geometric least-squares mean ratio; CI=Confidence interval - * 48.5 g fat
- † Median (Min, Max) reported for Tmax
Cmax (ng/mL) 14 935 (34) 16 1060 (25) 1.16 (0.96, 1.41) AUC0-72hr (hr∙ng/mL) 14 26200 (28) 16 38400 (18) 1.51 (1.33, 1.72) Tmax† (hr) 14 5.00
(3.00, 8.00)16 6.00
(5.00, 24.00)N/A Concomitant administration of posaconazole delayed-release tablets with drugs affecting gastric pH or gastric motility did not demonstrate any significant effects on posaconazole pharmacokinetic exposure (see Table 11).
Table 11: The Effect of Concomitant Medications that Affect the Gastric pH and Gastric Motility on the Pharmacokinetics of Posaconazole Delayed-Release Tablets in Healthy Volunteers Coadministered Drug Administration Arms Change in Cmax
(ratio estimate*; 90% CI of the ratio estimate)Change in AUC0-last
(ratio estimate*; 90% CI of the ratio estimate)- * Ratio Estimate is the ratio of coadministered drug plus posaconazole to posaconazole alone for Cmax or AUC0-last.
Mylanta® Ultimate strength liquid (Increase in gastric pH) 25.4 meq/5 mL, 20 mL ↑6%
(1.06; 0.90 -1.26)↑↑4%
(1.04; 0.90 -1.20)Ranitidine (Zantac®) (Alteration in gastric pH) 150 mg (morning dose of 150 mg Ranitidine BID) ↑4%
(1.04; 0.88 -1.23)↑↓3%
(0.97; 0.84 -1.12)Esomeprazole (Nexium®) (Increase in gastric pH) 40 mg (QAM 5 days, day -4 to 1) ↑2%
(1.02; 0.88-1.17)↑↑5%
(1.05; 0.89 -1.24)Metoclopramide (Reglan®) (Increase in gastric motility) 15 mg four times daily during 2 days (Day -1 and 1) ↓14%
(0.86, 0.73,1.02)↓7%
(0.93, 0.803,1.07)Posaconazole Oral Suspension
Posaconazole oral suspension is absorbed with a median Tmax of ~3 to 5 hours. Steady-state plasma concentrations are attained at 7 to 10 days following multiple-dose administration.
Following single-dose administration of 200 mg, the mean AUC and Cmax of posaconazole are approximately 3-times higher when the oral suspension is administered with a nonfat meal and approximately 4-times higher when administered with a high-fat meal (~50 gm fat) relative to the fasted state. Following single-dose administration of posaconazole oral suspension 400 mg, the mean AUC and Cmax of posaconazole are approximately 3-times higher when administered with a liquid nutritional supplement (14 gm fat) relative to the fasted state (see Table 12). In addition, the effects of varying gastric administration conditions on the Cmax and AUC of posaconazole oral suspension in healthy volunteers have been investigated and are shown in Table 13.
In order to assure attainment of adequate plasma concentrations, it is recommended to administer posaconazole oral suspension during or immediately following a full meal. In patients who cannot eat a full meal, posaconazole oral suspension should be taken with a liquid nutritional supplement or an acidic carbonated beverage (e.g., ginger ale).
Table 12: The Mean (%CV) [min-max] Posaconazole Pharmacokinetic Parameters Following Single-Dose Oral Suspension Administration of 200 mg and 400 mg Under Fed and Fasted Conditions Dose (mg) Cmax
(ng/mL)Tmax*
(hr)AUC (I)
(ng∙hr/mL)CL/F
(L/hr)t½
(hr)- * Median [min-max].
- † n=15 for AUC (I), CL/F, and t ½
- ‡ The subject with Tmax of 36 hrs had relatively constant plasma levels over 36 hrs (1.7 ng/mL difference between 4 hrs and 36 hrs).
- § n=10 for AUC (I), CL/F, and t ½
200 mg fasted
(n=20)†132 (50)
[45-267]3.50
[1.5-36‡]4179 (31)
[2705-7269]51 (25)
[28-74]23.5 (25)
[15.3-33.7]200 mg nonfat
(n=20)†378 (43)
[131-834]4 [3-5] 10,753 (35)
[4579-17,092]21 (39)
[12-44]22.2 (18)
[17.4-28.7]200 mg high fat
(54 gm fat)
(n=20)†512 (34)
[241-1016]5 [4-5] 15,059 (26)
[10,341-24,476]14 (24)
[8.2-19]23.0 (19)
[17.2-33.4]400 mg fasted
(n=23)§121 (75)
[27-366]4 [2-12] 5258 (48)
[2834-9567]91 (40)
[42-141]27.3 (26)
[16.8-38.9]400 mg with liquid nutritional supplement
(14 gm fat)
(n=23)§355 (43)
[145-720]5 [4-8] 11,295 (40)
[3865-20,592]43 (56)
[19-103]26.0 (19)
[18.2-35.0]Table 13: The Effect of Varying Gastric Administration Conditions on the Cmax and AUC of Posaconazole Oral Suspension in Healthy Volunteers* Study Description Administration Arms Change in Cmax
(ratio estimate†; 90% CI of the ratio estimate)Change in AUC
(ratio estimate†; 90% CI of the ratio estimate)- * In 5 subjects, the Cmax and AUC decreased substantially (range: -27% to -53% and -33% to -51%, respectively) when posaconazole was administered via an NG tube compared to when posaconazole was administered orally. It is recommended to closely monitor patients for breakthrough fungal infections when posaconazole is administered via an NG tube because a lower plasma exposure may be associated with an increased risk of treatment failure.
- † Ratio Estimate is the ratio of coadministered drug plus posaconazole to coadministered drug alone for Cmax or AUC.
- ‡ NG = nasogastric
400-mg single dose with a high-fat meal relative to fasted state (n=12) 5 minutes before high-fat meal ↑96%
(1.96; 1.48-2.59)↑111%
(2.11; 1.60-2.78)During high-fat meal ↑339%
(4.39; 3.32-5.80)↑382%
(4.82; 3.66-6.35)20 minutes after high-fat meal ↑333%
(4.33; 3.28-5.73)↑387%
(4.87; 3.70-6.42)400 mg BID and 200 mg QID for 7 days in fasted state and with liquid nutritional supplement (BOOST®) (n=12) 400 mg BID with BOOST ↑65%
(1.65; 1.29-2.11)↑66%
(1.66; 1.30-2.13)200 mg QID with BOOST No Effect No Effect Divided daily dose from 400 mg BID to 200 mg QID for 7 days regardless of fasted conditions or with BOOST (n=12) Fasted state ↑136%
(2.36; 1.84-3.02)↑161%
(2.61; 2.04-3.35)With BOOST ↑137%
(2.37; 1.86-3.04)↑157%
(2.57; 2.00-3.30)400-mg single dose with carbonated acidic beverage (ginger ale) and/or proton pump inhibitor (esomeprazole) (n=12) Ginger ale ↑92%
(1.92; 1.51-2.44)↑70%
(1.70; 1.43-2.03)Esomeprazole ↓32%
(0.68; 0.53-0.86)↓30%
(0.70; 0.59-0.83)400-mg single dose with a prokinetic agent (metoclopramide 10 mg TID for 2 days) + BOOST or an antikinetic agent (loperamide 4-mg single dose) + BOOST (n=12) With metoclopramide + BOOST ↓21%
(0.79; 0.72-0.87)↓19%
(0.81; 0.72-0.91)With loperamide + BOOST ↓3%
(0.97; 0.88-1.07)↑11%
(1.11; 0.99-1.25)400-mg single dose either orally with BOOST or via an NG tube with BOOST (n=16) Via NG tube‡ ↓19%
(0.81; 0.71-0.91)↓23%
(0.77; 0.69-0.86)Concomitant administration of posaconazole oral suspension with drugs affecting gastric pH or gastric motility results in lower posaconazole exposure. (See Table 14.)
Table 14: The Effect of Concomitant Medications that Affect the Gastric pH and Gastric Motility on the Pharmacokinetics of Posaconazole Oral Suspension in Healthy Volunteers Coadministered Drug (Postulated Mechanism of Interaction) Coadministered Drug Dose/Schedule Posaconazole Dose/Schedule Effect on Bioavailability of Posaconazole Change in Mean Cmax
(ratio estimate*; 90% CI of the ratio estimate)Change in Mean AUC
(ratio estimate*; 90% CI of the ratio estimate)- * Ratio Estimate is the ratio of coadministered drug plus posaconazole to coadministered drug alone for Cmax or AUC.
- † The tablet refers to a non-commercial tablet formulation without polymer.
- ‡ The drug interactions associated with the oral suspension are also relevant for the delayed-release tablet with the exception of Esomeprazole and Metoclopramide.
Cimetidine
(Alteration of gastric pH)400 mg BID × 10 days 200 mg (tablets) QD × 10 days† ↓ 39%
(0.61; 0.53-0.70)↓ 39%
(0.61; 0.54-0.69)Esomeprazole (Increase in gastric pH)‡ 40 mg QAM × 3 days 400 mg (oral suspension) single dose ↓ 46%
(0.54; 0.43-0.69)↓ 32%
(0.68; 0.57-0.81)Metoclopramide (Increase in gastric motility)‡ 10 mg TID × 2 days 400 mg (oral suspension) single dose ↓ 21%
(0.79; 0.72-0.87)↓ 19%
(0.81; 0.72-0.91)Distribution:
Posaconazole is highly bound to human plasma proteins (>98%), predominantly to albumin.
Metabolism:
Posaconazole primarily circulates as the parent compound in plasma. Of the circulating metabolites, the majority are glucuronide conjugates formed via UDP glucuronidation (phase 2 enzymes). Posaconazole does not have any major circulating oxidative (CYP450 mediated) metabolites. The excreted metabolites in urine and feces account for ~17% of the administered radiolabeled dose.
Posaconazole is primarily metabolized via UDP glucuronidation (phase 2 enzymes) and is a substrate for p-glycoprotein (P-gp) efflux. Therefore, inhibitors or inducers of these clearance pathways may affect posaconazole plasma concentrations. A summary of drugs studied clinically with the oral suspension or an early tablet formulation, which affect posaconazole concentrations, is provided in Table 15.
Table 15: Summary of the Effect of Coadministered Drugs on Posaconazole in Healthy Volunteers Coadministered Drug (Postulated Mechanism of Interaction) Coadministered Drug Dose/Schedule Posaconazole Dose/Schedule Effect on Bioavailability of Posaconazole Change in Mean Cmax
(ratio estimate*; 90% CI of the ratio estimate)Change in Mean AUC
(ratio estimate*; 90% CI of the ratio estimate)- * Ratio Estimate is the ratio of coadministered drug plus posaconazole to posaconazole alone for Cmax or AUC.
- † The tablet refers to a non-commercial tablet formulation without polymer.
Efavirenz
(UDP-G Induction)400 mg QD × 10 and 20 days 400 mg (oral suspension) BID × 10 and 20 days ↓45%
(0.55; 0.47-0.66)↓ 50%
(0.50; 0.43-0.60)Fosamprenavir (unknown mechanism) 700 mg BID × 10 days 200 mg QD on the 1st day, 200 mg BID on the 2nd day, then 400 mg BID × 8 Days ↓21%
0.79 (0.71-0.89)↓23%
0.77 (0.68-0.87)Rifabutin
(UDP-G Induction)300 mg QD × 17 days 200 mg (tablets) QD × 10 days† ↓ 43%
(0.57; 0.43-0.75)↓ 49%
(0.51; 0.37-0.71)Phenytoin
(UDP-G Induction)200 mg QD × 10 days 200 mg (tablets) QD × 10 days† ↓ 41%
(0.59; 0.44-0.79)↓ 50%
(0.50; 0.36-0.71)In vitro studies with human hepatic microsomes and clinical studies indicate that posaconazole is an inhibitor primarily of CYP3A4. A clinical study in healthy volunteers also indicates that posaconazole is a strong CYP3A4 inhibitor as evidenced by a >5-fold increase in midazolam AUC. Therefore, plasma concentrations of drugs predominantly metabolized by CYP3A4 may be increased by posaconazole. A summary of the drugs studied clinically, for which plasma concentrations were affected by posaconazole, is provided in Table 16 [see Contraindications (4) and Drug Interactions (7.1) including recommendations].
Table 16: Summary of the Effect of Posaconazole on Coadministered Drugs in Healthy Volunteers and Patients Coadministered Drug (Postulated Mechanism of Interaction is Inhibition of CYP3A4 by posaconazole) Coadministered Drug Dose/Schedule Posaconazole Dose/Schedule Effect on Bioavailability of Coadministered Drugs Change in Mean Cmax
(ratio estimate*; 90% CI of the ratio estimate)Change in Mean AUC
(ratio estimate*; 90% CI of the ratio estimate)- * Ratio Estimate is the ratio of coadministered drug plus posaconazole to coadministered drug alone for Cmax or AUC.
- † The tablet refers to a non-commercial tablet formulation without polymer.
- ‡ The mean terminal half-life of midazolam was increased from 3 hours to 7 to 11 hours during coadministration with posaconazole.
Sirolimus 2-mg single oral dose 400 mg (oral suspension) BID × 16 days ↑ 572%
(6.72; 5.62-8.03)↑ 788%
(8.88; 7.26-10.9)Cyclosporine Stable maintenance dose in heart transplant recipients 200 mg (tablets) QD × 10 days† ↑ cyclosporine whole blood trough concentrations
Cyclosporine dose reductions of up to 29% were requiredTacrolimus 0.05-mg/kg single oral dose 400 mg (oral suspension) BID × 7 days ↑ 121%
(2.21; 2.01-2.42)↑ 358%
(4.58; 4.03-5.19)Simvastatin 40-mg single oral dose 100 mg (oral suspension) QD × 13 days Simvastatin
↑ 841%
(9.41, 7.13-12.44)
Simvastatin Acid
↑ 817%
(9.17, 7.36-11.43)Simvastatin
↑ 931%
(10.31, 8.40-12.67)
Simvastatin Acid
↑634%
(7.34, 5.82-9.25)200 mg (oral suspension) QD × 13 days Simvastatin
↑ 1041%
(11.41, 7.99-16.29)
Simvastatin Acid
↑851%
(9.51, 8.15-11.10)Simvastatin
↑ 960%
(10.60, 8.63-13.02)
Simvastatin Acid
↑748%
(8.48, 7.04-10.23)Midazolam 0.4-mg single intravenous dose‡ 200 mg (oral suspension) BID × 7 days ↑ 30%
(1.3; 1.13-1.48)↑ 362%
(4.62; 4.02-5.3)0.4-mg single intravenous dose‡ 400 mg (oral suspension) BID × 7 days ↑62%
(1.62; 1.41-1.86)↑524%
(6.24; 5.43-7.16)2-mg single oral dose‡ 200 mg (oral suspension) QD × 7 days ↑ 169%
(2.69; 2.46-2.93)↑ 470%
(5.70; 4.82-6.74)2-mg single oral dose‡ 400 mg (oral suspension) BID × 7 days ↑ 138%
(2.38; 2.13-2.66)↑ 397%
(4.97; 4.46-5.54)Rifabutin 300 mg QD × 17 days 200 mg (tablets) QD × 10 days† ↑ 31%
(1.31; 1.10-1.57)↑ 72%
(1.72;1.51-1.95)Phenytoin 200 mg QD PO × 10 days 200 mg (tablets) QD × 10 days† ↑ 16%
(1.16; 0.85-1.57)↑ 16%
(1.16; 0.84-1.59)Ritonavir 100 mg QD × 14 days 400 mg (oral suspension) BID × 7 days ↑ 49%
(1.49; 1.04-2.15)↑ 80%
(1.8;1.39-2.31)Atazanavir 300 mg QD × 14 days 400 mg (oral suspension) BID × 7 days ↑ 155%
(2.55; 1.89-3.45)↑ 268%
(3.68; 2.89-4.70)Atazanavir/ ritonavir boosted regimen 300 mg/100 mg QD × 14 days 400 mg (oral suspension) BID × 7 days ↑ 53%
(1.53; 1.13-2.07)↑ 146%
(2.46; 1.93-3.13)Additional clinical studies demonstrated that no clinically significant effects on zidovudine, lamivudine, indinavir, or caffeine were observed when administered with posaconazole 200 mg QD; therefore, no dose adjustments are required for these coadministered drugs when coadministered with posaconazole 200 mg QD.
Excretion:
Following administration of posaconazole oral suspension, posaconazole is predominantly eliminated in the feces (71% of the radiolabeled dose up to 120 hours) with the major component eliminated as parent drug (66% of the radiolabeled dose). Renal clearance is a minor elimination pathway, with 13% of the radiolabeled dose excreted in urine up to 120 hours (<0.2% of the radiolabeled dose is parent drug).
Posaconazole delayed-release tablet is eliminated with a mean half-life (t½) ranging between 26 to 31 hours.
Posaconazole oral suspension is eliminated with a mean half-life (t½) of 35 hours (range: 20-66 hours).
12.4 Microbiology
Mechanism of Action:
Posaconazole blocks the synthesis of ergosterol, a key component of the fungal cell membrane, through the inhibition of cytochrome P-450 dependent enzyme lanosterol 14α-demethylase responsible for the conversion of lanosterol to ergosterol in the fungal cell membrane. This results in an accumulation of methylated sterol precursors and a depletion of ergosterol within the cell membrane thus weakening the structure and function of the fungal cell membrane. This may be responsible for the antifungal activity of posaconazole.
Resistance:
Clinical isolates of Candida albicans and Candida glabrata with decreased susceptibility to posaconazole were observed in oral swish samples taken during prophylaxis with posaconazole and fluconazole, suggesting a potential for development of resistance. These isolates also showed reduced susceptibility to other azoles, suggesting cross-resistance between azoles. The clinical significance of this finding is not known.
Antimicrobial Activity:
Posaconazole has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No drug-related neoplasms were recorded in rats or mice treated with posaconazole for 2 years at doses higher than the clinical dose. In a 2-year carcinogenicity study, rats were given posaconazole orally at doses up to 20 mg/kg (females), or 30 mg/kg (males). These doses are equivalent to 3.9- or 3.5-times the exposure achieved with a 400-mg BID oral suspension regimen, respectively, based on steady-state AUC in healthy volunteers administered a high-fat meal (400-mg BID oral suspension regimen). In the mouse study, mice were treated at oral doses up to 60 mg/kg/day or 4.8-times the exposure achieved with a 400-mg BID oral suspension regimen.
Posaconazole was not genotoxic or clastogenic when evaluated in bacterial mutagenicity (Ames), a chromosome aberration study in human peripheral blood lymphocytes, a Chinese hamster ovary cell mutagenicity study, and a mouse bone marrow micronucleus study.
Posaconazole had no effect on fertility of male rats at a dose up to 180 mg/kg (1.7 × the 400-mg BID oral suspension regimen based on steady-state plasma concentrations in healthy volunteers) or female rats at a dose up to 45 mg/kg (2.2 × the 400-mg BID oral suspension regimen).
13.2 Animal Toxicology and/or Pharmacology
In a nonclinical study using intravenous administration of posaconazole in very young dogs (dosed from 2 to 8 weeks of age), an increase in the incidence of brain ventricle enlargement was observed in treated animals as compared with concurrent control animals. No difference in the incidence of brain ventricle enlargement between control and treated animals was observed following the subsequent 5-month treatment-free period. There were no neurologic, behavioral or developmental abnormalities in the dogs with this finding, and a similar brain finding was not seen with oral posaconazole administration to juvenile dogs (4 days to 9 months of age).
-
14 CLINICAL STUDIES
14.1 Prophylaxis of Aspergillus and Candida Infections with Posaconazole Oral Suspension
Two randomized, controlled studies were conducted using posaconazole as prophylaxis for the prevention of invasive fungal infections (IFIs) among patients at high risk due to severely compromised immune systems.
The first study (Oral Suspension Study 1) was a randomized, double-blind trial that compared posaconazole oral suspension (200 mg three times a day) with fluconazole capsules (400 mg once daily) as prophylaxis against invasive fungal infections in allogeneic hematopoietic stem cell transplant (HSCT) recipients with Graft versus Host Disease (GVHD). Efficacy of prophylaxis was evaluated using a composite endpoint of proven/probable IFIs, death, or treatment with systemic antifungal therapy (patients may have met more than one of these criteria). This assessed all patients while on study therapy plus 7 days and at 16 weeks post-randomization. The mean duration of therapy was comparable between the 2 treatment groups (80 days, posaconazole; 77 days, fluconazole). Table 17 contains the results from Oral Suspension Study 1.
Table 17: Results from Blinded Clinical Study in Prophylaxis of IFI in All Randomized Patients with Hematopoietic Stem Cell Transplant (HSCT) and Graft-vs.-Host Disease (GVHD): Oral Suspension Study 1 Posaconazole
n=301Fluconazole
n=299- * Patients may have met more than one criterion defining failure.
- † Use of systemic antifungal therapy (SAF) criterion is based on protocol definitions (empiric/IFI usage >4 consecutive days).
- ‡ 95% confidence interval (posaconazole-fluconazole) = (-11.5%, +3.7%).
- § Patients who are lost to follow-up (not observed for 112 days), and who did not meet another clinical failure endpoint. These patients were considered failures.
On therapy plus 7 days Clinical Failure* 50 (17%) 55 (18%) Failure due to: Proven/Probable IFI 7 (2%) 22 (7%) (Aspergillus) 3 (1%) 17 (6%) (Candida) 1 (<1%) 3 (1%) (Other) 3 (1%) 2 (1%) All Deaths 22 (7%) 24 (8%) Proven/probable fungal infection prior to death 2 (<1%) 6 (2%) SAF† 27 (9%) 25 (8%) Through 16 weeks Clinical Failure*,‡ 99 (33%) 110 (37%) Failure due to: Proven/Probable IFI 16 (5%) 27 (9%) (Aspergillus) 7 (2%) 21 (7%) (Candida) 4 (1%) 4 (1%) (Other) 5 (2%) 2 (1%) All Deaths 58 (19%) 59 (20%) Proven/probable fungal infection prior to death 10 (3%) 16 (5%) SAF† 26 (9%) 30 (10%) Event free lost to follow-up§ 24 (8%) 30 (10%) The second study (Oral Suspension Study 2) was a randomized, open-label study that compared posaconazole oral suspension (200 mg 3 times a day) with fluconazole suspension (400 mg once daily) or itraconazole oral solution (200 mg twice a day) as prophylaxis against IFIs in neutropenic patients who were receiving cytotoxic chemotherapy for AML or MDS. As in Oral Suspension Study 1, efficacy of prophylaxis was evaluated using a composite endpoint of proven/probable IFIs, death, or treatment with systemic antifungal therapy (Patients might have met more than one of these criteria). This study assessed patients while on treatment plus 7 days and 100 days postrandomization. The mean duration of therapy was comparable between the 2 treatment groups (29 days, posaconazole; 25 days, fluconazole or itraconazole). Table 18 contains the results from Oral Suspension Study 2.
Table 18: Results from Open-Label Clinical Study 2 in Prophylaxis of IFI in All Randomized Patients with Hematologic Malignancy and Prolonged Neutropenia: Oral Suspension Study 2 Posaconazole
n=304Fluconazole/Itraconazole
n=298- * 95% confidence interval (posaconazole-fluconazole/itraconazole) = (-22.9%, -7.8%).
- † Patients may have met more than one criterion defining failure.
- ‡ Use of systemic antifungal therapy (SAF) criterion is based on protocol definitions (empiric/IFI usage >3 consecutive days).
- § Patients who are lost to follow-up (not observed for 100 days), and who did not meet another clinical failure endpoint. These patients were considered failures.
On therapy plus 7 days Clinical Failure*,† 82 (27%) 126 (42%) Failure due to: Proven/Probable IFI 7 (2%) 25 (8%) (Aspergillus) 2 (1%) 20 (7%) (Candida) 3 (1%) 2 (1%) (Other) 2 (1%) 3 (1%) All Deaths 17 (6%) 25 (8%) Proven/probable fungal infection prior to death 1 (<1%) 2 (1%) SAF‡ 67 (22%) 98 (33%) Through 100 days postrandomization Clinical Failure† 158 (52%) 191 (64%) Failure due to: Proven/Probable IFI 14 (5%) 33 (11%) (Aspergillus) 2 (1%) 26 (9%) (Candida) 10 (3%) 4 (1%) (Other) 2 (1%) 3 (1%) All Deaths 44 (14%) 64 (21%) Proven/probable fungal infection prior to death 2 (1%) 16 (5%) SAF‡ 98 (32%) 125 (42%) Event free lost to follow-up§ 34 (11%) 24 (8%) In summary, 2 clinical studies of prophylaxis were conducted with the posaconazole oral suspension. As seen in the accompanying tables (Tables 17 and 18), clinical failure represented a composite endpoint of breakthrough IFI, mortality and use of systemic antifungal therapy. In Oral Suspension Study 1 (Table 17), the clinical failure rate of posaconazole (33%) was similar to fluconazole (37%), (95% CI for the difference posaconazole–comparator -11.5% to 3.7%) while in Oral Suspension Study 2 (Table 18) clinical failure was lower for patients treated with posaconazole (27%) when compared to patients treated with fluconazole or itraconazole (42%), (95% CI for the difference posaconazole–comparator -22.9% to -7.8%).
All-cause mortality was similar at 16 weeks for both treatment arms in Oral Suspension Study 1 [POS 58/301 (19%) vs. FLU 59/299 (20%)]; all-cause mortality was lower at 100 days for posaconazole-treated patients in Oral Suspension Study 2 [POS 44/304 (14%) vs. FLU/ITZ 64/298 (21%)]. Both studies demonstrated substantially fewer breakthrough infections caused by Aspergillus species in patients receiving posaconazole prophylaxis when compared to patients receiving fluconazole or itraconazole.
14.2 Treatment of Oropharyngeal Candidiasis with Posaconazole Oral Suspension
Posaconazole Oral Suspension Study 3 was a randomized, controlled, evaluator-blinded study in HIV-infected patients with oropharyngeal candidiasis. Patients were treated with posaconazole or fluconazole oral suspension (both posaconazole and fluconazole were given as follows: 100 mg twice a day for 1 day followed by 100 mg once a day for 13 days).
Clinical and mycological outcomes were assessed after 14 days of treatment and at 4 weeks after the end of treatment. Patients who received at least 1 dose of study medication and had a positive oral swish culture of Candida species at baseline were included in the analyses (see Table 19). The majority of the subjects had C. albicans as the baseline pathogen.
Clinical success at Day 14 (complete or partial resolution of all ulcers and/or plaques and symptoms) and clinical relapse rates (recurrence of signs or symptoms after initial cure or improvement) 4 weeks after the end of treatment were similar between the treatment arms (see Table 19).
Mycologic eradication rates (absence of colony forming units in quantitative culture at the end of therapy, Day 14), as well as mycologic relapse rates (4 weeks after the end of treatment) were also similar between the treatment arms (see Table 19).
Table 19: Posaconazole Oral Suspension Clinical Success, Mycological Eradication, and Relapse Rates in Oropharyngeal Candidiasis Posaconazole Fluconazole Clinical Success at End of Therapy (Day 14) 155/169 (91.7%) 148/160 (92.5%) Clinical Relapse (4 Weeks after End of Therapy) 45/155 (29.0%) 52/148 (35.1%) Mycological Eradication (absence of CFU) at End of Therapy (Day 14) 88/169 (52.1%) 80/160 (50.0%) Mycological Relapse (4 Weeks after End of Treatment) 49/88 (55.6%) 51/80 (63.7%) Mycologic response rates, using a criterion for success as a posttreatment quantitative culture with ≤20 colony forming units (CFU/mL) were also similar between the two groups (posaconazole 68.0%, fluconazole 68.1%). The clinical significance of this finding is unknown.
14.3 Posaconazole Oral Suspension Treatment of Oropharyngeal Candidiasis Refractory to Treatment with Fluconazole or Itraconazole
Posaconazole Oral Suspension Study 4 was a noncomparative study of posaconazole oral suspension in HIV-infected subjects with OPC that was refractory to treatment with fluconazole or itraconazole. An episode of OPC was considered refractory if there was failure to improve or worsening of OPC after a standard course of therapy with fluconazole greater than or equal to 100 mg/day for at least 10 consecutive days or itraconazole 200 mg/day for at least 10 consecutive days and treatment with either fluconazole or itraconazole had not been discontinued for more than 14 days prior to treatment with posaconazole. Of the 199 subjects enrolled in this study, 89 subjects met these strict criteria for refractory infection.
Forty-five subjects with refractory OPC were treated with posaconazole oral suspension 400 mg BID for 3 days, followed by 400 mg QD for 25 days with an option for further treatment during a 3-month maintenance period. Following a dosing amendment, a further 44 subjects were treated with posaconazole 400 mg BID for 28 days. The efficacy of posaconazole was assessed by the clinical success (cure or improvement) rate after 4 weeks of treatment. The clinical success rate was 74.2% (66/89). The clinical success rates for both the original and the amended dosing regimens were similar (73.3% and 75.0%, respectively).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Delayed-Release Tablets
Posaconazole delayed-release tablets are available as yellow, coated, oblong, debossed with "100" on one side containing 100 mg of posaconazole. Bottles with child-resistant closures of 60 delayed-release tablets (NDC: 0254-2045-02). Store at 20-25°C (68-77°F), excursions permitted to 15-30°C (59-86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
17.1 Administration
Posaconazole Delayed-Release Tablets
Advise patients to take posaconazole delayed-release tablets with food.
Advise patients that posaconazole delayed-release tablets must be swallowed whole and not divided, crushed, or chewed.
Instruct patients that if they miss a dose, they should take it as soon as they remember. If they do not remember until it is within 12 hours of the next dose, they should be instructed to skip the missed dose and go back to the regular schedule. Patients should not double their next dose or take more than the prescribed dose.
17.2 Drug Interactions
Advise patients to inform their physician immediately if they:
- develop severe diarrhea or vomiting.
- are currently taking drugs that are known to prolong the QTc interval and are metabolized through CYP3A4.
- are currently taking a cyclosporine or tacrolimus, or they notice swelling in an arm or leg or shortness of breath.
- are taking other drugs or before they begin taking other drugs as certain drugs can decrease or increase the plasma concentrations of posaconazole.
17.3 Serious and Potentially Serious Adverse Reactions
Advise patients to inform their physician immediately if they:
- notice a change in heart rate or heart rhythm, or have a heart condition or circulatory disease. Posaconazole can be administered with caution to patients with potentially proarrhythmic conditions.
- are pregnant, plan to become pregnant, or are nursing.
- have liver disease or develop itching, nausea or vomiting, their eyes or skin turn yellow, they feel more tired than usual or feel like they have the flu.
- have ever had an allergic reaction to other antifungal medicines such as ketoconazole, fluconazole, itraconazole, or voriconazole.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 05/2019 Patient Information
Posaconazole delayed-release tabletsWhat is posaconazole?
Posaconazole delayed-release tablets are a prescription medicines used to help prevent fungal infections that can spread throughout your body (invasive fungal infections). These infections are caused by fungi called Aspergillus or Candida. Posaconazole is used in people who have an increased chance of getting these infections due to a weak immune system. These include people who have:- had a hematopoietic stem cell transplantation (bone marrow transplant) with graft versus host disease
- a low white blood cell count due to chemotherapy for blood cancers (hematologic malignancy)
It is not known if Posaconazole delayed-release tablets are safe and effective in children under 13 years of age.Who should not take posaconazole?
Do not take posaconazole if you:- are allergic to posaconazole, any of the ingredients in posaconazole, or other azole antifungal medicines. See the end of this leaflet for a complete list of ingredients in posaconazole.
- are taking any of the following medicines:
- sirolimus
- pimozide
- quinidine
- certain statin medicines that lower cholesterol (atorvastatin, lovastatin, simvastatin)
- ergot alkaloids (ergotamine, dihydroergotamine)
Do not start taking a new medicine without talking to your healthcare provider or pharmacist.What should I tell my healthcare provider before taking posaconazole?
Before you take posaconazole, tell your healthcare provider if you:- are taking certain medicines that lower your immune system like cyclosporine or tacrolimus.
- are taking certain drugs for HIV infection, such as ritonavir, atazanavir, efavirenz, or fosamprenavir. Efavirenz and fosamprenavir can cause a decrease in the posaconazole levels in your body. Efavirenz and fosamprenavir should not be taken with posaconazole.
- are taking midazolam, a hypnotic and sedative medicine.
- are taking vincristine, vinblastine and other "vinca alkaloids" (medicines used to treat cancer).
- have or had liver problems.
- have or had kidney problems.
- have or had an abnormal heart rate or rhythm, heart problems, or blood circulation problems.
- are pregnant or plan to become pregnant. It is not known if posaconazole will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if posaconazole passes into your breast milk. You and your healthcare provider should decide if you will take posaconazole or breastfeed. You should not do both.
Especially tell your healthcare provider if you take:- rifabutin or phenytoin. If you are taking these medicines, you should not take posaconazole delayed-release tablets.
Know the medicines you take. Keep a list of them with you to show your healthcare provider or pharmacist when you get a new medicine.How will I take posaconazole? - Do not switch between taking posaconazole delayed-release tablets and posaconazole oral suspension without talking to your healthcare provider.
- Take posaconazole exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much posaconazole to take and when to take it.
- Take posaconazole for as long as your healthcare provider tells you to take it.
- If you take too much posaconazole, call your healthcare provider or go to the nearest hospital emergency room right away.
-
Posaconazole delayed-release tablets:
- Take posaconazole delayed-release tablets with food.
- Take posaconazole delayed-release tablets whole. Do not break, crush, or chew posaconazole delayed-release tablets before swallowing. If you cannot swallow posaconazole delayed-release tablets whole, tell your healthcare provider. You may need a different medicine.
- If you miss a dose, take it as soon as you remember and then take your next scheduled dose at its regular time. If it is within 12 hours of your next dose, do not take the missed dose. Skip the missed dose and go back to your regular schedule. Do not double your next dose or take more than your prescribed dose.
What are the possible side effects of posaconazole?
Posaconazole may cause serious side effects, including:- drug interactions with cyclosporine or tacrolimus. If you take posaconazole with cyclosporine or tacrolimus, your blood levels of cyclosporine or tacrolimus may increase. Serious side effects can happen in your kidney or brain if you have high levels of cyclosporine or tacrolimus in your blood. Your healthcare provider should do blood tests to check your levels of cyclosporine or tacrolimus if you are taking these medicines while taking posaconazole. Tell your healthcare provider right away if you have swelling in your arm or leg or shortness of breath.
- problems with the electrical system of your heart (arrhythmias and QTc prolongation). Certain medicines used to treat fungus called azoles, including posaconazole, the active ingredient in posaconazole, may cause heart rhythm problems. People who have certain heart problems or who take certain medicines have a higher chance for this problem. Tell your healthcare provider right away if your heartbeat becomes fast or irregular.
- liver problems. Some people who also have other serious medical problems may have severe liver problems that may lead to death, especially if you take certain doses of posaconazole. Your healthcare provider should do blood tests to check your liver while you are taking posaconazole. Call your healthcare provider right away if you have any of the following symptoms of liver problems:
- itchy skin
- nausea or vomiting
- yellowing of your eyes
- feeling very tired
- flu-like symptoms
- increased amounts of midazolam in your blood. If you take posaconazole with midazolam, posaconazole increases the amount of midazolam in your blood. This can make your sleepiness last longer. Your healthcare provider should check you closely for side effects if you take midazolam with posaconazole.
- diarrhea
- nausea
- fever
- vomiting
- headache
- coughing
- low potassium levels in the blood
If you take posaconazole delayed-release tablets, tell your healthcare provider right away if you have diarrhea or vomiting.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of posaconazole. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store posaconazole? - Store posaconazole delayed-release tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Safely throw away medicine that is out of date or no longer needed.
General information about the safe and effective use of posaconazole.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use posaconazole for a condition for which it was not prescribed. Do not give posaconazole to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about posaconazole that is written for health professionals.What are the ingredients in posaconazole?
Active ingredient: posaconazole
Inactive ingredients:
Posaconazole delayed-release tablets: hypromellose acetate succinate, microcrystalline cellulose, hydroxypropylcellulose, silicon dioxide, croscarmellose sodium, magnesium stearate, and Opadry® II Yellow (consists of the following ingredients: polyvinyl alcohol partially hydrolyzed, Macrogol/PEG 3350, titanium dioxide, talc, and iron oxide yellow)
Manufactured for: Par Pharmaceutical, Chestnut Ridge, NY 10977, USA
Delayed-Release Tablets: Manuf. by: N. V. Organon, Kloosterstraat 6, 5349 AB Oss, Netherlands
usppi-gmk5592-t-1905r000
PI2045-01-78-01 -
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label
NDC: 0254-2045-02
Posaconazole
Delayed-Release
Tablets100 mg
Each tablet contains 100 mg posaconazole.
Attention: Posaconazole Oral Suspension and
Delayed-Release Tablets are NOT interchangeable
due to differences in the dosing of each formulation.Rx only
60 Tablets
PAR
PHARMACEUTICAL
-
INGREDIENTS AND APPEARANCE
POSACONAZOLE
posaconazole tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0254-2045 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength posaconazole (UNII: 6TK1G07BHZ) (posaconazole - UNII:6TK1G07BHZ) posaconazole 100 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE ACETATE SUCCINATE 06081224 (3 MM2/S) (UNII: 6N003M473W) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) silicon dioxide (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW (YELLOW (C48330)) Score no score Shape OVAL (oblong) Size 17mm Flavor Imprint Code 100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0254-2045-02 60 in 1 BOTTLE; Type 0: Not a Combination Product 08/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA205053 08/30/2019 Labeler - Par Pharmaceutical Inc. (092733690) Registrant - Merck Sharp & Dohme Corp. (001317601)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.