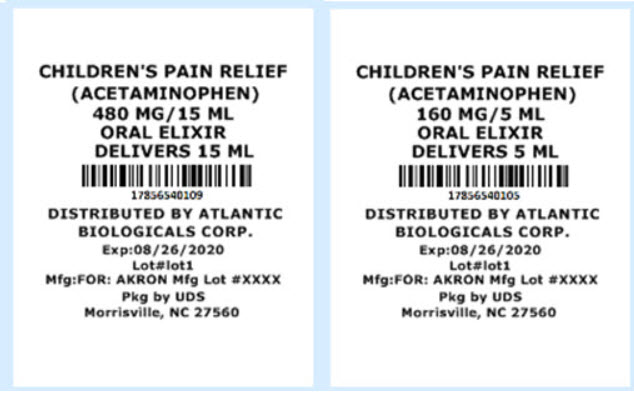
ACETAMINOPHEN by ATLANTIC BIOLOGICALS CORP. ACETAMINOPHEN elixir
ACETAMINOPHEN by
Drug Labeling and Warnings
ACETAMINOPHEN by is a Otc medication manufactured, distributed, or labeled by ATLANTIC BIOLOGICALS CORP.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each 5 mL = 1 teaspoonful)
- Purpose
- Uses
-
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if
- adult takes more than 6 doses in 24 hours, which is the maximum daily amount
- child takes more than 5 doses in 24 hours, which is the maximum daily amount taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks everyday while using this product.
Sore throat warning:
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or non-prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
Ask a doctor or pharmacist before use if your child is taking the blood thinning drug warfarin
When using this product: Do not exceed recommended dose
Stop use and ask a doctor if:
- Pain gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
- new symptoms occur.
- redness or swelling is present.
These could be signs of a serious condition.
Keep out of reach of children.
Overdose Warning:
taking more than the recommended dose (overdose) may cause liver damage.
In case of overdose get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults/children even if you do not notice any signs or symptoms.
-
Directions
- shake well before using
- find the right dose on chart below, if possible, use weight to dose; otherwise use age
- dosage may be repeated every 4 hours, or as directed by your doctor
- do not use more than 5 doses in 24 hours
- do not use more than 5 days unless directed by a doctor.
- find right dose on chart below, If possible, use weight to dose; otherwise, use age.
Weight (lbs.)
Age (years)
dosage-teaspoonful (tsp.)
under 24
under 2
consult Physician
24 to 35
2 to 3
1 tsp. (5 mL)
36 to 47
4 to 5
1 1/2 tsp. (7.5 mL)
48 to 59
6 to 8
2 tsp. (10 mL)
60 to 71
9 to 10
2 1/2 tsp. (12.5 mL)
72 to 95
11
3 tsp. (15 mL)
- Other information
-
Inactive Ingredients:
Grape Flavor, Citric Acid, Glycerin, Polyethylene Glycol 400, Purified Water, Sodium Citrate, Sodium Saccharin, Sorbitol Solution 70% & Sodium Benzoate.
How Supplied:
17856-5401-01 ACETAMINOPHEN - 48MG/1.5ML SYRINGE 120 UD
17856-5401-02 ACETAMINOPHEN - 64MG/2ML SYRINGE 120 UD
17856-5401-03 ACETAMINOPHEN - 96MG/3ML SYIRNGE 120 UD
17856-5401-04 ACETAMINOPHEN - 3.75 ML SYRINGE 48 ct UD
17856-5401-05 ACETAMINOPHEN - 5 ML CUP 72 ct UD
17856-5401-06 ACETAMINOPHEN - 192MG/6ML SYRINGE 48 ct UD
17856-5401-07 ACETAMINOPHEN - 240MG/7.5ML SYRINGE 48 ct UD
17856-5401-08 ACETAMINOPHEN - 325MG/10.15 ML CUP 72 ct UD
17856-5401-09 ACETAMINOPHEN - 480MG/15ML 50 CUP ct UD -
Questions or Comments?
Call (877) 225-6999 Monday - Friday 9AM-5PM EST
Manufactured for
Akron Pharma, Inc.,
Fairfeld, NJ - 07004Manufactured In USA
* This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Tylenol Elixir
Distributed by:
ATLANTIC BIOLOGICALS CORP.
Miami, FL 33179
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen elixirProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 17856-5401(NDC:71399-0161) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg in 5 mL Inactive Ingredients Ingredient Name Strength GRAPE (UNII: 6X543N684K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) WATER (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17856-5401-1 120 in 1 BOX, UNIT-DOSE 09/02/2020 1 1.5 mL in 1 SYRINGE; Type 0: Not a Combination Product 2 NDC: 17856-5401-2 120 in 1 BOX, UNIT-DOSE 09/02/2020 2 2 mL in 1 SYRINGE; Type 0: Not a Combination Product 3 NDC: 17856-5401-3 120 in 1 BOX, UNIT-DOSE 09/02/2020 3 3 mL in 1 SYRINGE; Type 0: Not a Combination Product 4 NDC: 17856-5401-4 48 in 1 BOX, UNIT-DOSE 09/02/2020 4 3.75 mL in 1 SYRINGE; Type 0: Not a Combination Product 5 NDC: 17856-5401-5 72 in 1 BOX, UNIT-DOSE 09/02/2020 5 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product 6 NDC: 17856-5401-6 48 in 1 BOX, UNIT-DOSE 09/02/2020 6 6 mL in 1 SYRINGE; Type 0: Not a Combination Product 7 NDC: 17856-5401-7 48 in 1 BOX, UNIT-DOSE 09/02/2020 7 7.5 mL in 1 SYRINGE; Type 0: Not a Combination Product 8 NDC: 17856-5401-8 72 in 1 BOX, UNIT-DOSE 09/02/2020 8 10.15 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product 9 NDC: 17856-5401-9 50 in 1 BOX, UNIT-DOSE 09/02/2020 9 15 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 07/28/2020 Labeler - ATLANTIC BIOLOGICALS CORP. (047437707) Establishment Name Address ID/FEI Business Operations ATLANTIC BIOLOGICALS CORP. 047437707 relabel(17856-5401) , repack(17856-5401)
Trademark Results [ACETAMINOPHEN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACETAMINOPHEN 85615223 not registered Dead/Abandoned |
General Merchandise importers and Expoters 2012-05-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.