HONEY BEE HYMENOPTERA VENOM VENOMIL DIAGNOSTIC kit HONEY BEE HYMENOPTERA VENOM VENOMIL MAINTENANCE kit WHITE FACED HORNET HYMENOPTERA VENOM VENOMIL DIAGNOSTIC kit WHITE FACED HORNET HYMENOPTERA VENOM VENOMIL MAINTENANCE kit YELLOW HORNET HYMENOPTERA VENOM VENOMIL DIAGNOSTIC kit YELLOW HORNET HYMENOPTERA VENOM VENOMIL MAINTENANCE kit WASP HYMENOPTERA VENOM VENOMIL DIAGNOSTIC kit WASP HYMENOPTERA VENOM VENOMIL MAINTENANCE kit YELLOW JACKET HYMENOPTERA VENOM VENOMIL DIAGNOSTIC kit YELLOW JACKET HYMENOPTERA VENOM VENOMIL MAINTENANCE kit MIXED VESPID HYMENOPTERA VENOM VENOMIL MAINTENANCE kit
Yellow Jacket Hymenoptera Venom Venomil Diagnostic by
Drug Labeling and Warnings
Yellow Jacket Hymenoptera Venom Venomil Diagnostic by is a Other medication manufactured, distributed, or labeled by Jubilant HollisterStier LLC, Jubilant HollisterStier LLC - HollisterStier Allergy. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
WARNINGS
This product is intended for use only by licensed medical personnel experienced in administering allergenic extracts and trained to provide immediate emergency treatment in the event of a life-threatening reaction.
Hymenoptera Venom extracts may potentially elicit a severe life-threatening systemic reaction, rarely resulting in death.(1) Therefore, emergency measures and personnel trained in their use must be available immediately in the event of such a reaction. Patients should be instructed to recognize adverse reaction symptoms, observed in the office for at least 30 minutes after skin testing or treatment, and cautioned to contact the physician's office if symptoms occur. See ADVERSE REACTION, Section 4, of this instruction for information regarding adverse event reporting.
All patients should have available an Emergency Anaphylaxis Kit containing epinephrine and be instructed in its use for emergency treatment of possible systemic reactions occurring at times after the patient has departed the testing or treatment premises. Patients with cardiovascular diseases and/or pulmonary diseases such as symptomatic unstable, steroid-dependent asthma, and/or those who are receiving cardiovascular drugs such as beta blockers, may be at higher risk for severe adverse reactions. These patients may also be more refractory to the normal allergy treatment regimen. Patients should be treated only if the benefit of treatment outweighs the risks.(1)
Patients on beta blockers may be more reactive to allergens given for testing or treatment and may be unresponsive to the usual doses of epinephrine used to treat allergic reactions.(2)
Immunotherapy for insect sting allergy should be given to those patients who have experienced significant systemic reactions (for detailed description of symptoms see INDICATIONS AND USAGE and ADVERSE REACTIONS) from insect stings and who demonstrate hypersensitivity by skin testing with these products. The only approved method for diagnosing insect sting allergic patients for immunization is by skin testing.
This product must never be injected intravenously.
Refer also to CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, ADVERSE REACTIONS and OVERDOSAGE for further discussion.
-
DESCRIPTION
Hymenoptera Venom Products available are sterile freeze-dried venom of Honey Bee (Apis mellifera) and venom protein of Yellow Jacket (Vespula sp.), Yellow Hornet (Dolichovespula arenaria), White-Faced Hornet (Dolichovespula maculata) and Wasp (Polistes sp.). Mixed Vespid venom protein (Yellow Jacket, Yellow Hornet and White-Faced Hornet) is also available.
The reconstituted single venom products are intended for subcutaneous injection for immunotherapy and percutaneous use for diagnosis. The Mixed Vespid venom protein is for immunotherapy only, not for diagnosis. Diagnosis should be based on individual venoms.
Because of the difficulty in collecting all species of Yellow Jacket and Wasp, the venom raw materials for these two insects may vary in species composition from lot to lot. A listing of the exact species content for any particular lot of Yellow Jacket or Wasp venom protein may be obtained by calling Technical Services at Jubilant HollisterStier, (800) 992-1120.Final containers of sterile freeze-dried venom products are sealed under vacuum. This will result in the diluting fluid being forcibly drawn into the sealed vial when the syringe needle penetrates the seal during reconstitution. See PRECAUTIONS.
Venom or venom protein is supplied in 2 mL diagnostic vials and in 2 mL vials for treatment maintenance. The chart below lists for each vial size the content of lyophilized venom or venom protein and reconstituted product, (mannitol and venom concentrations). Trace amounts of sodium chloride, potassium chloride, acetic acid and beta-alanine, as well as the constituents of the reconstituting fluid, will also be present.
Vial Size µg Venom or
Venom ProteinReconstitution mg/mL Mannitol Venom Concentration Single Venom
2 mL
120
1.2 mL
7.7 mg/mL
100µg/mL Mixed Vespid
2 mL
360
1.2 mL
23.1 mg/mL
300 µg/mL See product configuration in DOSAGE AND ADMINISTRATION Section.
Maintenance sterile freeze-dried products can be reconstituted in Sterile Albumin Saline with Phenol (which contains 0.9% NaCl, 0.4% phenol and 0.03% Human Serum Albumin) to a concentration of 100 µg/mL (300 µg/mL for Mixed Vespid venom protein). The diagnostic product should be reconstituted only with Sterile Albumin Saline with Phenol (0.4%). See DOSAGE AND ADMINISTRATION for details of dilutions for diagnosis and treatment.
Space is provided on the container label to record the date (month, day, year) venom is reconstituted. Refer to dating period shown under PRECAUTIONS. At the time of reconstitution, write the calculated reconstituted product expiration date (month, day, year) on the vial label in the space provided.
-
CLINICAL PHARMACOLOGY
Diagnosis
Diluted solutions of stinging insect venom injected intradermally will produce wheal and erythema reactions in patients who have significant IgE-mediated, Type I immediate hypersensitivity to stings of these insects.
Treatment
Repeated injections of increasing doses of insect venom extracts have been shown to ameliorate the intensity of allergic symptoms upon subsequent insect stings.(3, 4)
The mechanism by which hyposensitization is achieved is not known completely. IgG antibodies (blocking antibodies) appear in the serum of patients treated with injected venom. No direct relationship has been identified between the level of blocking antibody (or the ratio of blocking antibody to IgE antibody directed to the same venom antigens) and the degree of hyposensitization. However, patients who show protection from symptoms after stings have been found to have significant levels of specific blocking antibody.(3, 4)
Initially, after a period of immunotherapy with specific venom antigens, levels of IgE antibody may increase.(4)However, from studies carried out with other venom preparations, these levels are reported to decline after a time.(5) After maintenance level has been reached and maintained, symptoms after stings have been shown to decrease considerably.(3, 4)
It is not known if skin-sensitizing antibody can be eradicated or if the patient can be entirely cured, nor is it known how long immunotherapy must be continued.
In a clinical study with Jubilant HollisterStier venom products, injections (using the Suggested Dose Schedule under DOSAGE AND ADMINISTRATION) were given once per week at one study center, and twice or more per week at another center.(4) (For further discussion, see below). It must be considered important to achieve the 100 µg per venom maintenance dose (the maintenance dose for Mixed Vespid venom protein is 300 µg), since there are no data on effectiveness of maintenance levels below 100 µg per venom.
In the clinical trial, 97% of patients at the maintenance dosage (100 µg per venom) showed no systemic reaction following an insect sting challenge.(4)The remaining 3% had a milder reaction than noted prior to treatment. The patients in this study reached maintenance (100 µg per venom) usually within 2 1/2 -3 1/2 months after beginning therapy.(4)Whether efficacy of therapy is influenced by the time required to reach maintenance has not yet been determined.
Large local reactions occurred in approximately 60% of the patients given immunotherapy. Some form of systemic response occurred, often repeatedly, in one-third of the patients treated in the clinical trial.(4)Only one systemic response occurred on the first dose given. The rest occurred at various times in the course of immunotherapy. Some systemic manifestations may have occurred because of the patient's apprehension, and did not require treatment. Approximately one-fourth of the patients experiencing systemic responses were given some form of specific therapy (epinephrine, theophylline, or metaproteranol), some on several occasions.(4)
In deciding the criteria for proceeding from dose to dose of the Suggested Dose Schedule (see DOSAGE AND ADMINISTRATION), the results of the clinical study (4)should be considered. A study center "A" reporting the least number of systemic reactions during pre-maintenance treatment held the dose constant in most of the cases where significant local reactions occurred. With the systemic reactions reported, this center held the dose the same in approximately 80% of the incidences. The treatment injections were given at this center usually once per week, and if a patient missed an appointment, the next dose was often the same as the preceding dose (depending on the previous reactivity of the patient). Patients treated at this center reached maintenance in an average of 17-19 visits. Another study center "B", reporting a higher incidence of systemic reactions, was more regimented in following the Suggested Dose Schedule. This center reduced or held the dose the same in less than 10% of the cases reporting significant local reactions. With the systemic reactions reported, this center held the dose the same or reduced the dosage in approximately 20% of the cases. At this center, more than one injection per week was given at the outset as circumstances and sensitivity allowed. Patients treated at this center reached maintenance in an average of 14 visits.
Following the achievement of maintenance level (100 µg per venom), approximately 80% or more patients were given a second maintenance injection at a 1-week interval. The third maintenance injection was usually (in approximately 60% of the patients) at a 2-week interval. The next injection was usually within 3 weeks, and thereafter, the patients were injected for ongoing maintenance at approximately monthly intervals.(4) -
INDICATIONS AND USAGE
Insect stings may induce a wide range of allergic symptoms in sensitive patients. A normal sting response is initial burning or stinging pain that may be intense and last several minutes to an hour or more. There is usually some local swelling coming on immediately and persisting for several days. The location of the sting has considerable influence on the intensity of the pain and extent of swelling. Stings on the fingers or feet produce much pain, but less swelling; whereas a sting on the head or face produces extensive swelling with variable pain.
Local reactions coming on rapidly and larger than the usual local reaction, particularly if the swelling spans both adjacent joints on the extremities, can indicate hypersensitivity. Systemic symptoms come on shortly after the sting, often within seconds to minutes. Symptoms may range from generalized flushing, itching, redness, diffuse swelling of the skin or urticarial wheals, abdominal cramps, nausea, vomiting, or incontinence of urine or stool, to faintness, blurring or loss of vision, unconsciousness, seizures, respiratory or cardiac arrest, or death. Later reactions may consist of fever, achiness, malaise, joint swelling, urticaria or other signs of vascular damage typical of serum sickness, a Type III reaction. Typical delayed Type IV reactions may also occur.(6) Rarely, other types of severe reactions to insect stings have been reported.(6)These include serum sickness, hematologic abnormalities, and neurological disorders commencing some time after a sting, and not associated with anaphylactoid reactions. These patients are not candidates for immunotherapy using insect venoms.
(1) Diagnosis
Skin testing with insect venoms is useful to demonstrate the presence of IgE antibodies which account for the patient's symptoms.(3)Patients are seldom able to identify the insect which stung them, so skin testing is used to determine the insect culprit. Dilutions of these venom products will help judge the sensitivity of the patient and whether the patient should be treated.(7)
It is not absolutely known what levels (micrograms) of venom, that elicit positive skin tests, are diagnostic of clinical sensitivity. However, patients with a history of reactions (any of three types: generalized urticaria or angioedema; respiratory difficulty due either to laryngeal edema or to bronchospasm; or vascular collapse, with or without loss of consciousness) to previous stings and a positive skin test to a venom intradermal injection of approximately 1 µg/mL had about a 60% chance of reacting again when stung by the same insect. These patients should receive venom immunotherapy.(3)
Patients with a history of reaction (any of the three reaction types described above) to previous stings, but who did not demonstrate a positive skin test reaction to venom, were considered in a previous study not to be clinically sensitive, and were not treated.(3) We cannot recommend treatment for such patients.
Another study demonstrated false positive reactions when skin testing with venom concentrations of 10 µg/mL and 100 µg/mL was carried out.(8) Thus there can be a nonspecific skin test reaction potentially due to the pharmacological action of the venom at higher concentrations.
The best statement that can be made, at present, is that patients with significant positive history (reactions of the three types described above) following an insect sting, and who do react with a positive skin test to a venom concentration of 1 µg/mL or less, are recommended for treatment. Patients who have the history described above, but who do not react to a 1 µg/mL intradermal venom skin test, cannot be recommended for treatment. At present, the data does not exist, to determine whether a patient who might react to a higher concentration, e.g., 2-10 µg/mL, is at risk from a subsequent sting or not. Since it is not known if sting-sensitive patients who subsequently lose their IgE anti-venom antibody can be resensitized by further stings, it is advisable to retest these patients after any subsequent stings.(3) However, since the level of venom-specific IgE may fall to low levels briefly after a sting, patients should not be re-tested until 2 to 4 weeks after any sting.
(2) Treatment
Immunotherapy is indicated for those patients diagnosed as sensitive (see Diagnosis above) and is accomplished by using graduated dilutions of the appropriate insect venom or venoms to control the severity of the patient's symptoms from subsequent stings.
Increasing doses of venom are given at intervals, dependent on the patient's ability to tolerate the venoms, until a maintenance dosage (100 µg per venom is recommended - 300 µg in the case of the Mixed Vespid venom protein) is reached and maintained.
Venom sensitivity differs for individual patients, thus it is not possible to provide a dosage schedule that is universally suited to all patients. The dosage schedule shown under DOSAGE AND ADMINISTRATION is a summary of the schedule used in clinical trials of our product and found suitable for the majority of patients.
In highly sensitive patients, the physician may be required to use a modified dose schedule, based on the patient's sensitivity to and tolerance of the injections. Lower initial doses and smaller dosage increments than shown under DOSAGE AND ADMINISTRATION may be necessary.
-
CONTRAINDICATIONS
There are no known absolute contraindications to immunotherapy using Hymenoptera Venom Products. See also PRECAUTIONS and WARNINGS.
Patients showing negative intradermal skin tests to specific venoms at 1 µg/mL are not recommended for venom treatment.
Any injections, including immunotherapy, should be avoided in patients with a bleeding tendency. Patients with cardiovascular diseases and/or pulmonary diseases such as symptomatic unstable, steroid-dependent asthma, and/or those who are receiving cardiovascular drugs such as beta blockers, may be at higher risk for severe adverse reactions. These patients may also be more refractory to the normal allergy treatment regimen. Patients should be treated only if the benefit of treatment outweighs the risks.(1)
Patients on beta blockers may be more reactive to allergens given for testing or treatment and maybe unresponsive to the usual doses of epinephrine used to treat systemic reactions.(2)
Since there are differences of opinion concerning the possibility of routine immunizations exacerbating autoimmune diseases, immunotherapy should be given cautiously to patients with other immunologic diseases and only if the risk from insect stings is greater than the risk of exacerbating the underlying disorder.
-
WARNINGS
See WARNINGS box at the beginning of this Instruction Sheet. See also PRECAUTIONS.
Venom extract must be temporarily withheld from patients or the dose adjusted downward if any of the following conditions exist: (1) severe symptoms of rhinitis and/or asthma; (2) infection or flu accompanied by fever; (3) any evidence of an excessively large local or any generalized reaction during the initial stages of immunotherapy, or during maintenance therapy; and/or (4) insect sting prior to a scheduled injection. Do not administer venom injections during a period of symptoms following an insect sting or on the day the patient received an insect sting, since this could result in an allergen load that exceeds the patient's tolerance.
THE CONCENTRATE MUST NOT BE INJECTED AT ANY TIME UNLESS TOLERANCE HAS BEEN ESTABLISHED. DILUTE CONCENTRATED EXTRACTS WITH STERILE ALBUMIN SALINE WITH PHENOL (0.4%) FOR SKIN TESTING AND IMMUNOTHERAPY.
INJECTIONS MUST NEVER BE GIVEN INTRA VENOUSLY. Subcutaneous injection is recommended. Intracutaneous or intramuscular injections may produce large local reactions or be excessively painful. AFTER INSERTING NEEDLE SUBCUTANEOUSLY, BUT BEFORE INJECTING, ALWAYS WITHDRAW THE PLUNGER SLIGHTLY. IF BLOOD APPEARS IN THE SYRINGE, CHANGE NEEDLE AND GIVE THE INJECTION IN ANOTHER SITE.
Patients with hypersensitivity to insect venom who undergo desensitization treatment while under concomitant therapy with ACE (angiotensin-converting enzyme) inhibitors, may have an increased risk of life-threatening anaphylactic reactions.(9) Patients without insect venom hypersensitivity, who take ACE inhibitors, and are stung by insects such as bee or wasp can show such reactions as well.(10)
Two patients undergoing desensitization treatment with Hymenoptera Venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.(11)IF CHANGING TO A DIFFERENT LOT OR A FRESHLY RECONSTITUTED VIAL OF VENOM EXTRACT:
All extracts lose potency over time, and a fresh extract could have an effective potency that is substantially greater than that of the old extract. The first dose from the new vial should not exceed 50% of the previous dose.
IF THE VENOM EXTRACT PREVIOUSLY USED WAS FROM ANOTHER MANUFACTURER: Since manufacturing processes and sources of raw materials differ among manufacturers, the interchangeability of extracts from different manufacturers cannot be insured. The starting dose of the venom extract therefore should be greatly decreased even though the extract is the same formula and dilution. In general, a dose reduction to 50% of the previous product dose should be adequate, but each situation must be evaluated separately considering the patient's history of sensitivity, tolerance of previous injections, and other factors. If the patient tolerates a 50% decrease, the next dose could be raised to the previous dose amount. If the decrease is greater than 50%, the next dose would need to be determined by the allergist, depending on the situation. Dose intervals should not exceed one week when rebuilding dose. See DOSAGE AND ADMINISTRATION.
IF A PROLONGED PERIOD OF TIME HAS ELAPSED SINCE THE LAST INJECTION: Patients may lose tolerance for allergen injections during prolonged periods between doses. The duration of tolerance is an individual characteristic and varies from patient to patient. In general, the longer the lapse in the injection schedule, the greater dose reduction required. If the interval since last dose is over four weeks, perform skin tests to determine starting dose. See DOSAGE AND ADMINISTRATION.
IF THE PREVIOUS EXTRACT WAS OUTDATED: The dating period for allergenic extracts indicates the time that they can be expected to remain potent under refrigerated storage conditions (2° - 8°C). During the storage of extracts, even under ideal conditions, some loss of potency occurs. For this reason, extracts should not be used beyond their expiration date. If a patient has been receiving injections of an outdated extract, s/he may experience excessive local or systemic reactions when changed to a new, and possibly more potent extract. In general, the longer the material has been outdated, the greater the dose reduction necessary when starting the fresh extract.Proper selection of the dose and careful injection should prevent most systemic reactions. It must be remembered, however, that allergenic extracts are highly potent in sensitive individuals and that systemic reactions of varying degrees of severity may occur, ranging from mild to life-threatening anaphylaxis, or even death, as described under INDICATIONS AND USAGE and ADVERSE REACTIONS. Patients should be informed of this, and the warnings and precautions should be discussed prior to immunotherapy. See PRECAUTIONS below. Systemic reactions should be treated as indicated in ADVERSE REACTIONS.
-
PRECAUTIONS
1. GENERAL
The presence of asthmatic signs and symptoms appear to be an indicator for severe reactions following allergy injections. An assessment of airway obstruction either by measurement of peak flow or an alternate procedure may provide a useful indicator as to the advisability of administering an allergy injection. (1, 12-16)
Concentrated extracts must not be injected unless tolerance has been established.
Diluting fluid should be forcibly drawn into the sealed vial when the syringe needle penetrates the seal during reconstitution. Failure of this to occur for a particular vial indicates possible loss of vacuum. Vials without vacuum should be returned to the manufacturer.
Record date of reconstitution and expiration date of reconstituted product in the space provided on the product label. Date of expiration after reconstitution must not exceed the Final Expiration Date indicated on the container label. (See table below for expiration dates, including dilutions).
Store freeze-dried and reconstituted venom product, stock solutions and venom dilutions constantly at 2 - 8 C.
Venom Concentration
Diluent
Recommended Expiration Date* 100 µg/mL
Albumin Saline with Phenol (0.4%)
6 Months
10µg/mL
Albumin Saline with Phenol (0.4%)
1 month
1 µg/mL
Albumin Saline with Phenol (0.4%)
1 month
0.1 µg/mL
Albumin Saline with Phenol (0.4%)
14 days
Less than 0.1 µg/mL
Albumin Saline with Phenol (0.4%)
Prepare fresh daily
*But not to exceed Final Expiration Date indicated on the container label.
Sterile solutions, vials, syringes, etc., should be used and aseptic precautions observed in making dilutions.
To avoid cross-contamination, do not use the same needle to withdraw materials from vials of more than one extract, or extract followed by diluent.
A sterile tuberculin syringe, with a needle at least 5/8" long and graduated in 0.01 mL units, should be used to measure carefully each dose from the appropriate dilution. Aseptic techniques should always be employed when injections are being administered.
A separate sterile syringe should be used for each patient to prevent transmission of hepatitis and other infectious agents from one person to another.
Patient reactions to previous injections should be reviewed before each new injection so that dose can be adjusted accordingly. See ADVERSE REACTIONS and WARNINGS.
Rarely, a patient is encountered who develops systemic reactions to minute doses of allergen and does not demonstrate increasing tolerance to injections after several months of treatment. It is suggested that if systemic reactions or excessive local responses occur persistently at very small doses, efforts at immunotherapy should be stopped.
PATIENTS SHOULD BE OBSERVED IN THE OFFICE FOR AT LEAST 30 MINUTES AFTER SKIN TESTING AND AFTER EACH TREATMENT INJECTION. Most severe reactions will occur within this time period, and rapid treatment measures should be instituted. See ADVERSE REACTIONS for such treatment measures.2. INFORMATION FOR PATIENTS
Patients should be instructed in the recognition of adverse reactions to immunotherapy, and in particular, to the symptoms of shock. (See WARNINGS box at the beginning of this Instruction Sheet). Patients should be made to understand the importance of a 30 minute observation period following skin testing or therapeutic injections, and be cautioned to return to the office promptly if symptoms occur after leaving. Patients should be instructed in the use of, and have available, an Emergency Anaphylaxis Kit for self-administration of epinephrine.
Patients must be instructed to report any insect stings that have occurred, since a venom injection should not be given on the same day as the sting, nor during a time when the patient is still experiencing symptoms from the sting.3. DRUG INTERACTIONS
Patients with cardiovascular diseases and/or pulmonary diseases such as symptomatic, unstable, steroid-dependent asthma, and/or those who are receiving cardiovascular drugs such as beta blockers, may be at higher risk for severe adverse reactions. These patients may also be more refractory to the normal allergy treatment regimen. Patients should be treated only if the benefit of treatment outweighs the risks.(1)
Patients on beta blockers may be more reactive to allergens given for testing or treatment and may be unresponsive to the usual doses of epinephrine used to treat allergic reactions.
See WARNINGS section regarding concurrent treatment with ACE inhibitors.
Certain medications may lessen the skin test wheal and erythema responses elicited by allergens and histamine for varying time periods. Conventional antihistamines should be discontinued at least 5 days before skin testing. Long acting antihistamines should be discontinued for at least 3 weeks prior to skin testing.(17) Topical steroids should be discontinued at the skin test site for at least 2-3 weeks before skin testing.(17, 18)
Tricyclic antidepressants such as doxepin, should be withheld for at least 7 days before skin testing.(19) Topical local anesthetics may suppress the flare responses and should be avoided on skin test sites.(20)
When using other drugs in patients receiving allergenic extracts, always consult the product labeling of the other drugs to determine any possible interaction with use of allergenic extracts, and specifically with stinging insect (Hymenoptera) venom extracts.
4. CARCINOGENESIS AND MUTAGENSIS AND IMPAIRMENT OF FERTILITY
Long-term studies in animals have not been conducted with allergenic extracts to determine their potential for carcinogenicity, mutagenicity, or impairment of fertility.
5. PREGNANCY
(12, 21)
Animal reproduction studies have not been conducted with Hymenoptera Venom Products. It is also not known whether Hymenoptera Venom Products can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Hymenoptera Venom Products should be given to a pregnant woman only if clearly needed.On the basis of histamine's known ability to contract uterine muscle, theoretically, a systemic reaction, whether occurring from insect sting or from venom skin testing or treatment dose, should be avoided. Therefore, the physician must carefully consider the benefit-to-risk ratio, to both patient and fetus, of continuing venom immunotherapy during pregnancy, or performing venom skin testing, and especially of initiating a venom immunotherapy program where there is a possibility that the patient may not be able to reach the recommended maintenance dose without significant risk of a systemic reaction.
6. NURSING MOTHERS
There are no current studies on secretion of the allergenic extract components in human milk or effect on the nursing infant. Because many drugs are excreted in human milk, caution should be exercised when allergenic extracts are administered to a nursing woman.
7. PEDIATRIC USE
Since dosage for the pediatric population is the same as for adults, the larger volumes of solution may produce excessive discomfort. Therefore, in order to achieve the total dose required, the volume of the dose may need to be divided into more than one injection per visit. A study done in children ages 4 to 17 showed no special problems with venom immunotherapy in this population.(22)
8. GERIATRIC USE
The reactions from immunotherapy can be expected to be the same in elderly patients as in younger ones. Elderly patients may be more likely to be on medication that could block the effect of epinephrine which could be used to treat serious reactions, or they could be more sensitive to the cardiovascular side effect of epinephrine because of pre-existing cardiovascular disease.(23)
-
ADVERSE REACTIONS
Physicians administering Hymenoptera Venom testing or treatment materials should be experienced in the treatment of severe systemic reactions (see WARNINGS box at the beginning of this Instruction Sheet).
(1) Local Reactions
Some erythema, swelling or pruritis at the site of injection are common, the extent varying with the patient. Excessively large, painful or persistent local reactions can occur from skin tests or immunotherapy. Frequent application of cold, wet dressings to the area and/or the use of oral antihistamines will ameliorate the discomfort. Reactions usually subside in 24-36 hours. Large local reactions occurred in approximately 60% of the patients given immunotherapy in a clinical study. None of the local reactions required specific treatment; however, subsequent injections in many instances were held to the previous dose or a reduced dose. Some patients had repeated large local reactions that slowed the increase in the immunotherapy dose.4
See CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION Sections.
A mild burning immediately after the injection is to be expected. This usually leaves in 10 to 20 seconds. See also WARNINGS and PRECAUTIONS regarding proper method and route of injection.
(2) Systemic Reactions
Most severe systemic reactions will begin within a 30 minute time period, but systemic reactions may occur at any time after skin tests or immunotherapy. Symptoms may range from mild to life threatening from anaphylaxis as described under INDICATIONS AND USAGE.
With careful attention to dosage and administration, severe systemic reactions occur infrequently, but it cannot be overemphasized that in sensitive individuals, any injection could result in anaphylactic shock. Therefore, it is imperative that physicians administering allergenic extracts understand and be prepared for the treatment of severe reactions. See CLINICAL PHARMACOLOGY for clinical incidence of systemic reactions and course of action following these reactions.
If a systemic or anaphylactic reaction does occur, inject 1:1000 epinephrine-hydrochloride intramuscularly or subcutaneously.
EPINEPHRINE DOSAGE
ADULT: 0.3 to 0.5 mL should be injected. Repeat in 5 to 10 minutes if necessary.
PEDIATRIC: The usual initial dose is 0.01 mg (mL) per kg body weight or 0.3 mg (mL) per square meter of body surface area. Suggested dosage for infants to 2 years of age is 0.05 mL to 0.1 mL; for children 2 to 6 years, 0.15 mL; and children 6 to 12 years, 0.2 mL. Single pediatric doses should not exceed 0.3 mg (mL). Doses may be repeated as frequently as every 20 minutes, depending on the severity of the condition and the response of the patient.
After administration of epinephrine, profound shock or vasomotor collapse should be treated with intravenous fluids, and possibly vasoactive drugs. Airway patency should be insured. Oxygen should be given by mask. Intravenous antihistamines, inhaled bronchodilators, theophylline and/or corticosteroids may be used if necessary after adequate epinephrine and circulatory support have been given.
Emergency resuscitation measures and personnel trained in their use must be available immediately in the event of a serious systemic or anaphylactic reaction not responsive to the above measures [Ref. J. Allergy and Clinical Immunology, 77(2): p.271-273, 1986]. Rarely are all of the above measures necessary; epinephrine usually produces a prompt response. However, the physician should be prepared in advance for all contingencies. Promptness in beginning emergency treatment measures is of utmost importance. For recommendations regarding how to proceed with venom extract dose following systemic reactions, see WARNINGS, PRECAUTIONS and DOSAGE AND ADMINISTRATION.
3. Adverse Event Reporting
Report all adverse events to Jubilant HollisterStier LLC Customer Technical Services Department at 1(800) 992-1120. A voluntary adverse event reporting system for health professionals is available through the FDA MEDWATCH program. Preprinted forms (FDA Form 3500) are available from the FDA by calling 1(800) FDA-1088. Completed forms should be mailed to MEDWATCH, 5600 Fisher Lane, Rockville, MD 20852-9787 or Fax to: 1(800) FDA-0178. - OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
(1) General
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Reconstitute and dilute the freeze-dried venom as directed below. Sterile Albumin Saline with Phenol (0.4%) must be used to reconstitute and dilute the venoms for skin testing and treatment.
Reconstitute the freeze-dried venoms by adding 1.2 mL Sterile Albumin Saline with Phenol (0.4%) to the vial using a sterile syringe. Swirl or rock the container to dissolve the venom completely. DO NOT SHAKE, since shaking can cause foaming.
Dilutions (see table below) must be made in Sterile Albumin Saline with Phenol (0.4%). They must be made accurately and aseptically, using sterile solutions, vials, syringes, etc., and thoroughly mixed by rocking or swirling. DO NOT SHAKE. Maintain stock solutions and dilutions constantly at 2° - 8°C.Extract of Volume Extract Concentration
Diluent Volume
Dilution Concentration 1 part of
100 µg/mL
+
9 parts
=
10 µg/mL
1 part of
10 µg/mL
+
9 parts
=
1 µg/mL
1 part of
1µg/mL
+
9 parts
=
0.1µg/mL
1 part of
0.1 µg/mL
+
9 parts
=
0.01 µg/mL
1 part of
0.01 µg/mL
+
9 parts
=
0.001 µg/mL
1 part of
0.001 µg/mL
+
9 parts
=
0.0001 µg/mL
As an example of the above dilution table:
Extract of Volume Extract Concentration
Diluent Volume
Dilution Concentration 0.2mL of
100 µg/mL
+
1.8mL
=
10 µg/mL
0.2mL of
10 µg/mL
+
1.8mL
=
1 µg/mL
0.2mL of
1 µg/mL
+
1.8mL
=
0.1 µg/mL
0.2mL of
0.1 µg/mL
+
1.8mL
=
0.01 µg/mL
0.2mL of
0.01 µg/mL
+
1.8mL
=
0.001 µg/mL
0.2mL of
0.001 µg/mL
+
1.8mL
=
0.0001 µg/mL
NOTE: Mixed Vespid Venom Protein concentrations will be three times that shown above.
USE OF VENOMIL DIAGNOSTIC SETS
The Venomil Diagnostic Sets from Jubilant HollisterStier contain a vial of freeze dried venom protein that when reconstituted as instructed below will contain 100 µg venom or venom protein/mL.
To use the Venomil Diagnostic set, follow these steps:
1. Open box and remove contents. Be sure to read the complete package Instruction Sheet paying particular attention to the WARNINGS, PRECAUTIONS, CONTRAINDICATIONS, and ADVERSE REACTIONS.
2. Remove the freeze-dried venom vial and the vial of diluent provided with the kit. Withdraw 1.3 mL of Albumin Saline with Phenol (0.4%) from the diluent vial using a 2 or 3 mL disposable syringe. Expel some Albumin Saline with Phenol (0.4%) from the syringe until exactly 1.2 mL are remaining in the syringe. The remaining Albumin Saline with Phenol (0.4%) in the diluent vial may be marked "Control" and used as a negative control for prick testing.
3. Insert the needle of the diluent syringe into the vial of venom and expel the diluent. Remove the syringe. Swirl or rock the vial to dissolve the venom completely. DO NOT SHAKE. Shaking can cause foaming of the extract.
At this point, you have completed the reconstitution of the freeze-dried venom. The reconstituted products contain 100 µg of venom or venom protein per mL. DO NOT USE THIS STRENGTH FOR INTRADERMAL SKIN TESTING. DISCARD AFTER THE DILUTIONS HAVE BEEN PREPARED.
4. Remove six vial labels from the kit and mark them: 10 µg/mL, 1 µg/mL, 0.1 µg/mL, 0.01 µg/mL, 0.001 µg/mL and 0.0001 µg/mL. Withdraw 0.2 mL of venom extract in a 1 mL syringe from the vial reconstituted in step #3. Insert the syringe needle into one vial of 1.8 mL Albumin Saline with Phenol (0.4%). Slowly expel the 0.2 mL venom into it. Swirl or rock to mix, and label 10 µg/mL.
5. Withdraw 0.2 mL of the 10 µg/mL venom extract and inject into another vial of 1.8 mL Albumin Saline with Phenol (0.4%). Mix and label 1 µg/mL.
6. The four additional dilutions should be prepared in the same manner.(2) Diagnosis
Since the level of insect venom specific IgE may fall to low levels briefly after a reaction to a sting, patients should not be tested until 2 to 4 weeks after any sting. Skin testing should be carried out with all five individual venoms, since many patients have multiple sensitivities.(4) Mixed Vespid venom protein should be used only for therapy - not for diagnosis.
Prick testing should be done before intradermal testing to determine appropriate concentration for intradermal testing. See Intradermal Tests. Skin testing (prick and intradermal) provides information to assist in identifying those patients who are to be classified as extremely sensitive and who may not tolerate the Suggested Dose Schedule. See DOSAGE AND ADMINISTRATION, Immunotherapy CAUTION.
In both the prick and intradermal tests, a negative control test with diluent alone must be performed. A histamine positive control test is also recommended.
The flexor surface of the forearm is the usual location for skin testing. It is important that a separate sterile syringe and needle be used for each extract and each patient.
Prick Tests: Prick tests are accomplished by applying one drop of the 1 µg/mL venom extract to the forearm, and by pricking the skin through the surface of the drop with a sterile 27 gauge needle. The prick is superficial and should not draw blood.
Skin response should be assessed after approximately 15-20 minutes.
For prick tests, a positive reaction (reaction greater than diluent control) at the 1 µg/mL concentration indicates a high level of sensitivity to the test venom.
Intradermal Tests: Patients showing a positive reaction to the prick test at the 1 µg/mL concentration should begin intradermal tests at concentrations of not more than 0.0001 to 0.001 µg/mL. Patients with negative prick tests may begin intradermal tests at a concentration of 0.001 µg/mL.
A 1 mL tuberculin syringe with a short 27-gauge needle should be used to deliver a volume of 0.05 mL for intradermal testing. Introduce the needle into the superficial skin layers, bevel down, until the bevel is completely buried, then slowly inject a 0.05 mL aliquot of the venom dilution, making a small bleb.
Start intradermal tests with the most dilute solution. If after 20 minutes no skin reaction is obtained, continue the intradermal testing using ten-fold increments in the concentration until a reaction of 5-10 mm wheal and 11-20 mm erythema is obtained, or until a concentration of 1 µg/mL has been tested, whichever occurs first.
A patient should be considered sensitive to the test venom when a skin response of 5-10 mm wheal and 11-20 mm erythema (or greater) occurs at a concentration of 1 µg/mL or less,(8)providing that this reaction is greater than that of the diluent control.
(3) Immunotherapy
For proper method and route of injection, see WARNINGS, PRECAUTIONS and ADVERSE REACTIONS.
The most common site of injection is the lateral aspect of the upper arm.
Patients who have multiple venom sensitivities should be given each specific venom injection in a separate site. (Except, if the patient has sensitivities to Yellow Jacket, Yellow Hornet, and White-Faced Hornet venoms concurrently, s/he can be injected with Mixed Vespid venom protein, an equal mixture of these three vespid venoms). Note which venom preparation is injected at a specific site, so that dosage of that venom preparation can be adjusted if an excessive local reaction occurs. In patients receiving more than one venom, there is theoretically a greater risk of systemic reactions.
CAUTION: Sensitivity to venom differs from patient to patient. Thus, it is not possible to provide a dosage schedule suitable for all patients. The Suggested Dose Schedule shown below was used in
clinical trials(4)and should be suitable for a majority of patients.
IN EXTREMELY SENSITIVE PATIENTS, however, an individualized dose schedule must be employed which will be dictated by the patient's sensitivity. This individualized schedule will probably include weaker dilutions and smaller increments between doses in progressing to the maintenance level (100 µg per venom).
In identifying those patients to be classified as extremely sensitive, individuals reacting with significant skin test (wheal greater than 5 mm and erythema greater than 20 mm) at intradermal skin test concentrations of 0.01 µg/mL or less, or those patients experiencing a systemic reaction to any venom skin test concentration, should be considered highly sensitive.Suggested Dose Schedule for a Single Venom:
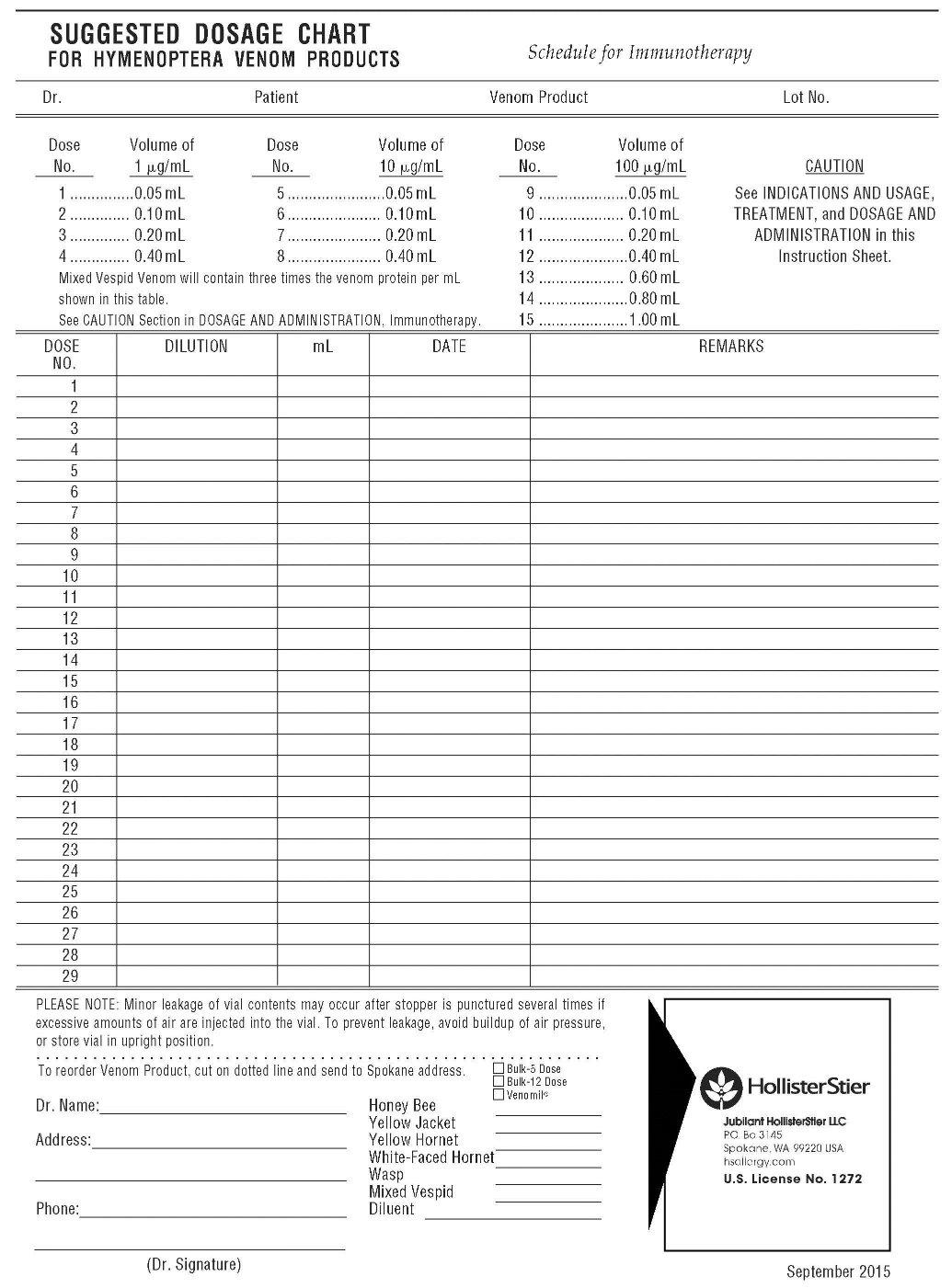
Dose No. *Volume of 1 µg/mL Dose No. Volume of 10 µg/mL Dose No. Volume of 100 µg/mL 1..... ...0.05 mL 5..... ...0.05 mL 9..... ...0.05mL 2..... ...0.10 mL 6..... ...0.10 mL 10.... ...0.10mL 3..... ...0.20 mL 7..... ...0.20 mL 11.... ...0.20mL 4..... ...0.40 mL 8..... ...0.40 mL 12.... ...0.40mL 13.... ...0.60mL 14.... ...0.80mL 15.... ...1.00mL Mixed Vespid Venom will contain three times the venom protein per mL shown in this table.
*See preceding CAUTION Section.ALTERNATE MAINTENANCE DOSE SCHEDULE
If the above suggested dosage schedule has been followed, Dose #15 will have emptied the third vial of venom. There should now be three vials of freeze-dried venom remaining in the maintenance set. If a smaller volume maintenance dose is desired, then the remaining vials of venom may be reconstituted with 0.6 mL of Sterile Albumin Saline with Phenol (0.4%) instead of the previously recommended 1.2 mL. When 0.6 mL is used for reconstitution, the maintenance dose volume then becomes 0.5 mL instead of 1.0 mL. The 0.5 mL injection will still contain 100 micrograms of venom or venom protein.
Precautions should be taken to ensure that maintenance level injections of 0.5 mL are given only from those vials of venom that have been reconstituted with 0.6 mL of diluting fluid. Any other volume used for reconstitution will not give 100 micrograms of venom or venom protein at a dosage of 0.5 mL In proceeding with the Suggested Dose Schedule, or modified schedules (for highly sensitive patients) it is recommended that injections be given at least once per week, as in the clinical studies. (See CLINICAL PHARMACOLOGY and INDICATIONS AND USAGE). When building the dose, it is important that dose intervals not exceed one week since longer intervals may decrease the patient's tolerance of the extract.
Based on the clinical studies (4)it is suggested that if a systemic, extremely large local (10 cm or more in duration, or other severe local symptoms), or persistent and severe delayed local reaction occurs during the dose building phase, the dose at the next visit be held constant (or reduced, depending on judgment of the severity of the reaction) as was done at Study Center "A," which reported the least number of systemic reactions during the course of therapy.
It must be considered important to achieve the 100 µg per venom maintenance dose (the maintenance dose for Mixed Vespid venom protein is 300 µg), since there are no data on effectiveness of maintenance levels below 100 µg per venom. Following the achievement of maintenance level (100 µg per venom), it is recommended that a second maintenance injection be given at a 1-week interval, and a third maintenance injection at a 2-week interval. Administer the next injection at a 3-week interval, and then monthly for ongoing maintenance.
See CLINICAL PHARMACOLOGY and INDICATIONS AND USAGE for further information regarding clinical studies on which the above recommendations are based.
The optimum duration for immunotherapy is not known, so current recommendations are that maintenance injections be continued indefinitely, year around, particularly in patients experiencing life-threatening anaphylaxis after insect stings.GERIATRIC USE
The dose for elderly patients is the same as for adult patients under 65.(23) (See PRECAUTIONS).
-
HOW SUPPLIED
Jubilant HollisterStier LLC sterile freeze-dried Hymenoptera Venom Products are supplied in vacuum-sealed vials containing venom extract and excipients: mannitol (for Vespid Venom Protein), and mannitol and sodium chloride (for Honey Bee Venom). (See the chart under DESCRIPTION or the latest Allergy Product Price List for vial sizes and content). Reconstituting fluid [Sterile Albumin Saline with Phenol (0.4%)] is supplied with the Venomil® kits, and is also available separately. (Note: Diagnostic kits also contain Sterile Empty Vials.)
Storage:
Store freeze-dried and reconstituted venom product, and venom dilutions, at 2° - 8° C, and keep at this temperature range during office use. -
LIMITED WARRANTY
A number of factors beyond our control could reduce the efficacy of this product or even result in an ill effect following its use. These include storage and handling of the product after it leaves our hands, diagnosis, dosage, method of administration and biological differences in individual patients. Because of these factors, it is important that this product be stored properly and that the directions be followed carefully during use.
No warranty, express or implied, including any warranty of merchantability or fitness, is made. Representatives of the Company are not authorized to vary the terms or the contents of any printed labeling, including the package insert, for this product except by printed notice from the Company's headquarters. The prescriber and user of this product must accept the terms hereof. -
REFERENCES
1. Lockey, Richard F., Linda M. Benedict, Paul C. Turkeltaub, Samuel C. Bukantz. Fatalities from immunotherapy (IT) and skin testing (ST). J. Allergy Clin. Immunol. 79 (4): 660-677, 1987.
2. Jacobs, Robert L., Goeffrey W. Rake, Jr., et. al. Potentiated anaphylaxis in patients with drug-induced beta-adrenergic blockade. J. Allergy Clin. Immunol. 68 (2): 125-127, August 1981.
3. Hunt, K. J., M. D. Valentine, A. K. Sobotka, A. W. Benton, F. J. Amodio, L. M. Lichtenstein. A controlled trial of immunotherapy in insect hypersensitivity. New Eng. J. Med. 299: 157-161, July 27, 1978.
4. Summary of data from BB-IND 1292 clinical studies, 1978-79, on Hollister-Stier products.
5. Amodio, F., L. Markley, M. D. Valentine, A. K. Sobotka, L. M. Lichtenstein. Maintenance immunotherapy for Hymenoptera sensitivity. J. Allergy Clin. Immunol. 61 (3): 134, 1978.
6. Reisman, R. E., Allergy Principles and Practice. E. Middleton, C. E. Reed, E. F. Ellis, ed. C. V. Mosby Co., 1978.
7. Sobotka, A. K., N. F. Adkinson, Jr., M. D. Valentine, L. M. Lichtenstein. Allergy to insect stings. IV. Diagnosis by R.A.S.T. J. Immunol. 121 (6): 2477-2484, 1978.
8. Hunt, K. J., M. D. Valentine, A. K. Sobotka, L. M. Lichtenstein. Diagnosis of allergy to stinging insects by skin testing with Hymenoptera venoms. Annals Int. Med. 85: 56-59, 1976.
9. Annals of Allergy, Asthma and Immunology. Inhibitors of angiotensin II: Potential hazards for patients at risk for anaphylaxis. Editorial. 78: 527-529, June 1997.
10. Pharm. Ind. (Germany). Anaphylactoid reactions in patients treated with ACE inhibitor treatment in combination with desensitization treatment or after insect bites. 56 (9): IX226-227, 1994.
11. Tunon-De-Lara, J. M., et al. ACE inhibitors and anaphylactoid reactions during venom immunotherapy. The Lancet (United Kingdom). 340 (8824): 908, Oct. 10, 1992.
12. Weinstien, A. M., B. D. Dubin, W. K. Podleski, S. L. Spector, R. S. Farr. Asthma and pregnancy. JAMA. 124 (11): 1161-1165, 1979.
13. Reid, M. J., R. F. Lockey, P. C. Turkletaub, T. A. E. Platts-Mills. Survey of fatalities from skin testing and immunotherapy. J. Allergy Clin. Immunol. 92 (1): 6-15, July 1993.
14. Reid, M. J., G. Gurka. Deaths associated with skin testing and immunotherapy. J. Allergy Clin. Immunol. 97 (1) Part 3:231, Abstract 195, January 1996.
15. Thompson, R. A. et al. Report of a WHO/IUIS working group. The current status of allergen immunotherapy (hyposensitization). Allergy. 44: 369-379, 1989.
16. Malling, H.J., B. Weeke, et al. The European Academy of Allergology and Clinical Immunology. Position Papers. Allergy. 48 (Supplement 14): 9-82, 1993.
17. Pipkorn, Ulf. Pharmacological influence of anti-allergic medication on In Vivo allergen testing. Allergy. 43: 81-86, 1988.
18. Andersson, M. U. Pipkorn. Inhibition of the dermal immediate allergic reaction through prolonged treatment with topical glucocorticosteroids. J. Allergy Clin. Immunol. 79 (2): 345-349, February 1987.
19. Rao, Kamineni S., et al. Duration of suppressive effect of tricyclic anti-depressants on histamine induced wheal and flare reactions on human skin. J. Allergy Clin. Immunol. 82: 752-757, November 1988.
20. Pipkorn, Ulf, M. Andersson. Topical dermal anesthesia inhibits the flare but not the wheal response to allergen and histamine in the skin prick test. Clinical Allergy. 17: 307-311, 1987.
21. DuBuske, L. M., C. J. Ling, A. L. Sheffer. Special problems regarding allergy immunotherapy. Immunol. Allergy Clin. North Am. (USA). 12 (1): 145-175, 1992.
22. Graft, D., K. Schuberth, A. Kagey-Sobotka, K. Kwiterovich, Y. Niv, L. Lichtenstein, M. Valentine. Assessment of prolonged venom immunotherapy in children. J. Allergy Clin. Immunol. 80 (2): 162-169, August 1987.
23. Peebles, Ray Stokes, Jr., B. Bochner, Howard J. Zeitz, ed. Anaphylaxis in the elderly. Immunol. Allergy Clin. of North Am. 13 (3): 627-646, August 1993. - SUGGESTED DOSAGE CHART
- Honey Bee Venomil Diagnostic Image
- Honey Bee Venomil Diagnostic Carton Label
- Honey Bee Venomil Maintenance Image
- Honey Bee Venomil Maintenance Carton Label
- Mixed Vespid Venomil Maintenance Image
- Mixed Vespid Venomil Maintenance Carton Label
- Wasp Venomil Diagnostic Image
- Wasp Venomil Diagnostic Carton Label
- Wasp Venomil Maintenance Image
- Wasp Venomil Maintenance Carton Label
- White Faced Hornet Venomil Diagnostic Image
- White-Faced Hornet Venomil Diagnostic Carton Label
- White Faced Hornet Venomil Maintenance Image
- White-Faced Hornet Venomil Maintenance Carton Label
- Yellow Hornet Venomil Diagnostic Image
- Yellow Hornet Venomil Diagnostic Carton Label
- Yellow Hornet Venomil Maintenance Image
- Yellow Hornet Venomil Maintenance Carton Label
- Yellow Jacket Venomil Diagnostic Image
- Yellow Jacket Venomil Diagnostic Carton Label
- Yellow Jacket Venomil Maintenance Image
- Yellow Jacket Venomil Maintenance Carton Label
-
INGREDIENTS AND APPEARANCE
HONEY BEE HYMENOPTERA VENOM VENOMIL DIAGNOSTIC
honey bee hymenoptera venom venomil diagnostic kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 65044-9960 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9960-1 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1.2 mL Part 1 of 1 HONEY BEE HYMENOPTERA VENOM
honey bee hymenoptera venom injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 65044-9980 Route of Administration PERCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA VENOM (UNII: 76013O881M) (APIS MELLIFERA VENOM - UNII:76013O881M) APIS MELLIFERA VENOM 100 ug in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9980-2 1.2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103882 02/22/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103882 02/22/1982 HONEY BEE HYMENOPTERA VENOM VENOMIL MAINTENANCE
honey bee hymenoptera venom venomil maintenance kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 65044-9970 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9970-1 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 VIAL 7.2 mL Part 1 of 1 HONEY BEE HYMENOPTERA VENOM
honey bee hymenoptera venom injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 65044-9980 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA VENOM (UNII: 76013O881M) (APIS MELLIFERA VENOM - UNII:76013O881M) APIS MELLIFERA VENOM 100 ug in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9980-2 1.2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103882 02/22/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103882 02/22/1982 WHITE FACED HORNET HYMENOPTERA VENOM VENOMIL DIAGNOSTIC
white faced hornet hymenoptera venom venomil diagnostic kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 65044-9961 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9961-1 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1.2 mL Part 1 of 1 WHITE FACED HORNET HYMENOPTERA VENOM
white faced hornet hymenoptera venom injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 65044-9981 Route of Administration PERCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOLICHOVESPULA MACULATA VENOM PROTEIN (UNII: J8DAZ3T66L) (DOLICHOVESPULA MACULATA VENOM PROTEIN - UNII:J8DAZ3T66L) DOLICHOVESPULA MACULATA VENOM PROTEIN 100 ug in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ACETIC ACID (UNII: Q40Q9N063P) ALANINE (UNII: OF5P57N2ZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9981-2 1.2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103885 02/22/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103885 02/22/1982 WHITE FACED HORNET HYMENOPTERA VENOM VENOMIL MAINTENANCE
white faced hornet hymenoptera venom venomil maintenance kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 65044-9971 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9971-1 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 VIAL 7.2 mL Part 1 of 1 WHITE FACED HORNET HYMENOPTERA VENOM
white faced hornet hymenoptera venom injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 65044-9981 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOLICHOVESPULA MACULATA VENOM PROTEIN (UNII: J8DAZ3T66L) (DOLICHOVESPULA MACULATA VENOM PROTEIN - UNII:J8DAZ3T66L) DOLICHOVESPULA MACULATA VENOM PROTEIN 100 ug in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ACETIC ACID (UNII: Q40Q9N063P) ALANINE (UNII: OF5P57N2ZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9981-2 1.2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103885 02/22/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103885 02/22/1982 YELLOW HORNET HYMENOPTERA VENOM VENOMIL DIAGNOSTIC
yellow hornet hymenoptera venom venomil diagnostic kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 65044-9962 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9962-1 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1.2 mL Part 1 of 1 YELLOW HORNET HYMENOPTERA VENOM
yellow hornet hymenoptera venom injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 65044-9982 Route of Administration PERCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOLICHOVESPULA ARENARIA VENOM PROTEIN (UNII: 7PI26E943G) (DOLICHOVESPULA ARENARIA VENOM PROTEIN - UNII:7PI26E943G) DOLICHOVESPULA ARENARIA VENOM PROTEIN 100 ug in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ACETIC ACID (UNII: Q40Q9N063P) ALANINE (UNII: OF5P57N2ZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9982-2 1.2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103886 02/22/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103886 02/22/1982 YELLOW HORNET HYMENOPTERA VENOM VENOMIL MAINTENANCE
yellow hornet hymenoptera venom venomil maintenance kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 65044-9972 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9972-1 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 VIAL 7.2 mL Part 1 of 1 YELLOW HORNET HYMENOPTERA VENOM
yellow hornet hymenoptera venom injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 65044-9982 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOLICHOVESPULA ARENARIA VENOM PROTEIN (UNII: 7PI26E943G) (DOLICHOVESPULA ARENARIA VENOM PROTEIN - UNII:7PI26E943G) DOLICHOVESPULA ARENARIA VENOM PROTEIN 100 ug in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ACETIC ACID (UNII: Q40Q9N063P) ALANINE (UNII: OF5P57N2ZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9982-2 1.2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103886 02/22/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103886 02/22/1982 WASP HYMENOPTERA VENOM VENOMIL DIAGNOSTIC
wasp hymenoptera venom venomil diagnostic kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 65044-9963 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9963-1 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1.2 mL Part 1 of 1 WASP HYMENOPTERA VENOM
wasp hymenoptera venom injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 65044-9983 Route of Administration PERCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLISTES ANNULARIS VENOM PROTEIN (UNII: 987GS3GJZX) (POLISTES ANNULARIS VENOM PROTEIN - UNII:987GS3GJZX) POLISTES ANNULARIS VENOM PROTEIN 25 ug in 1 mL POLISTES EXCLAMANS VENOM PROTEIN (UNII: L0L5D5D9BQ) (POLISTES EXCLAMANS VENOM PROTEIN - UNII:L0L5D5D9BQ) POLISTES EXCLAMANS VENOM PROTEIN 25 ug in 1 mL POLISTES FUSCATUS VENOM PROTEIN (UNII: AKT0E6058K) (POLISTES FUSCATUS VENOM PROTEIN - UNII:AKT0E6058K) POLISTES FUSCATUS VENOM PROTEIN 25 ug in 1 mL POLISTES METRICUS VENOM PROTEIN (UNII: ZN217W5Y50) (POLISTES METRICUS VENOM PROTEIN - UNII:ZN217W5Y50) POLISTES METRICUS VENOM PROTEIN 25 ug in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ACETIC ACID (UNII: Q40Q9N063P) ALANINE (UNII: OF5P57N2ZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9983-2 1.2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103884 02/22/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103884 02/22/1982 WASP HYMENOPTERA VENOM VENOMIL MAINTENANCE
wasp hymenoptera venom venomil maintenance kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 65044-9973 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9973-1 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 VIAL 7.2 mL Part 1 of 1 WASP HYMENOPTERA VENOM
wasp hymenoptera venom injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 65044-9983 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLISTES ANNULARIS VENOM PROTEIN (UNII: 987GS3GJZX) (POLISTES ANNULARIS VENOM PROTEIN - UNII:987GS3GJZX) POLISTES ANNULARIS VENOM PROTEIN 25 ug in 1 mL POLISTES EXCLAMANS VENOM PROTEIN (UNII: L0L5D5D9BQ) (POLISTES EXCLAMANS VENOM PROTEIN - UNII:L0L5D5D9BQ) POLISTES EXCLAMANS VENOM PROTEIN 25 ug in 1 mL POLISTES FUSCATUS VENOM PROTEIN (UNII: AKT0E6058K) (POLISTES FUSCATUS VENOM PROTEIN - UNII:AKT0E6058K) POLISTES FUSCATUS VENOM PROTEIN 25 ug in 1 mL POLISTES METRICUS VENOM PROTEIN (UNII: ZN217W5Y50) (POLISTES METRICUS VENOM PROTEIN - UNII:ZN217W5Y50) POLISTES METRICUS VENOM PROTEIN 25 ug in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ACETIC ACID (UNII: Q40Q9N063P) ALANINE (UNII: OF5P57N2ZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9983-2 1.2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103884 02/22/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103884 02/22/1982 YELLOW JACKET HYMENOPTERA VENOM VENOMIL DIAGNOSTIC
yellow jacket hymenoptera venom venomil diagnostic kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 65044-9964 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9964-1 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 1.2 mL Part 1 of 1 YELLOW JACKET HYMENOPTERA VENOM
yellow jacket hymenoptera venom injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 65044-9984 Route of Administration PERCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VESPULA GERMANICA VENOM PROTEIN (UNII: 8SH7583MUK) (VESPULA GERMANICA VENOM PROTEIN - UNII:8SH7583MUK) VESPULA GERMANICA VENOM PROTEIN 20 ug in 1 mL VESPULA MACULIFRONS VENOM PROTEIN (UNII: V34908RT03) (VESPULA MACULIFRONS VENOM PROTEIN - UNII:V34908RT03) VESPULA MACULIFRONS VENOM PROTEIN 20 ug in 1 mL VESPULA PENSYLVANICA VENOM PROTEIN (UNII: Q79PS8P34R) (VESPULA PENSYLVANICA VENOM PROTEIN - UNII:Q79PS8P34R) VESPULA PENSYLVANICA VENOM PROTEIN 20 ug in 1 mL VESPULA SQUAMOSA VENOM PROTEIN (UNII: D7974DM2EJ) (VESPULA SQUAMOSA VENOM PROTEIN - UNII:D7974DM2EJ) VESPULA SQUAMOSA VENOM PROTEIN 20 ug in 1 mL VESPULA VULGARIS VENOM PROTEIN (UNII: S125N1F5X5) (VESPULA VULGARIS VENOM PROTEIN - UNII:S125N1F5X5) VESPULA VULGARIS VENOM PROTEIN 20 ug in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ACETIC ACID (UNII: Q40Q9N063P) ALANINE (UNII: OF5P57N2ZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9984-2 1.2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103887 02/22/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103887 02/22/1982 YELLOW JACKET HYMENOPTERA VENOM VENOMIL MAINTENANCE
yellow jacket hymenoptera venom venomil maintenance kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 65044-9974 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9974-1 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 VIAL 7.2 mL Part 1 of 1 YELLOW JACKET HYMENOPTERA VENOM
yellow jacket hymenoptera venom injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 65044-9984 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VESPULA GERMANICA VENOM PROTEIN (UNII: 8SH7583MUK) (VESPULA GERMANICA VENOM PROTEIN - UNII:8SH7583MUK) VESPULA GERMANICA VENOM PROTEIN 20 ug in 1 mL VESPULA MACULIFRONS VENOM PROTEIN (UNII: V34908RT03) (VESPULA MACULIFRONS VENOM PROTEIN - UNII:V34908RT03) VESPULA MACULIFRONS VENOM PROTEIN 20 ug in 1 mL VESPULA PENSYLVANICA VENOM PROTEIN (UNII: Q79PS8P34R) (VESPULA PENSYLVANICA VENOM PROTEIN - UNII:Q79PS8P34R) VESPULA PENSYLVANICA VENOM PROTEIN 20 ug in 1 mL VESPULA SQUAMOSA VENOM PROTEIN (UNII: D7974DM2EJ) (VESPULA SQUAMOSA VENOM PROTEIN - UNII:D7974DM2EJ) VESPULA SQUAMOSA VENOM PROTEIN 20 ug in 1 mL VESPULA VULGARIS VENOM PROTEIN (UNII: S125N1F5X5) (VESPULA VULGARIS VENOM PROTEIN - UNII:S125N1F5X5) VESPULA VULGARIS VENOM PROTEIN 20 ug in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ACETIC ACID (UNII: Q40Q9N063P) ALANINE (UNII: OF5P57N2ZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9984-4 1.2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103887 02/22/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103887 02/22/1982 MIXED VESPID HYMENOPTERA VENOM VENOMIL MAINTENANCE
mixed vespid hymenoptera venom venomil maintenance kitProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 65044-9975 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9975-1 1 in 1 TRAY; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 VIAL 7.2 mL Part 1 of 1 MIXED VESPID HYMENOPTERA VENOM
mixed vespid hymenoptera venom injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 65044-9985 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOLICHOVESPULA MACULATA VENOM PROTEIN (UNII: J8DAZ3T66L) (DOLICHOVESPULA MACULATA VENOM PROTEIN - UNII:J8DAZ3T66L) DOLICHOVESPULA MACULATA VENOM PROTEIN 100 ug in 1 mL DOLICHOVESPULA ARENARIA VENOM PROTEIN (UNII: 7PI26E943G) (DOLICHOVESPULA ARENARIA VENOM PROTEIN - UNII:7PI26E943G) DOLICHOVESPULA ARENARIA VENOM PROTEIN 100 ug in 1 mL VESPULA GERMANICA VENOM PROTEIN (UNII: 8SH7583MUK) (VESPULA GERMANICA VENOM PROTEIN - UNII:8SH7583MUK) VESPULA GERMANICA VENOM PROTEIN 20 ug in 1 mL VESPULA MACULIFRONS VENOM PROTEIN (UNII: V34908RT03) (VESPULA MACULIFRONS VENOM PROTEIN - UNII:V34908RT03) VESPULA MACULIFRONS VENOM PROTEIN 20 ug in 1 mL VESPULA PENSYLVANICA VENOM PROTEIN (UNII: Q79PS8P34R) (VESPULA PENSYLVANICA VENOM PROTEIN - UNII:Q79PS8P34R) VESPULA PENSYLVANICA VENOM PROTEIN 20 ug in 1 mL VESPULA SQUAMOSA VENOM PROTEIN (UNII: D7974DM2EJ) (VESPULA SQUAMOSA VENOM PROTEIN - UNII:D7974DM2EJ) VESPULA SQUAMOSA VENOM PROTEIN 20 ug in 1 mL VESPULA VULGARIS VENOM PROTEIN (UNII: S125N1F5X5) (VESPULA VULGARIS VENOM PROTEIN - UNII:S125N1F5X5) VESPULA VULGARIS VENOM PROTEIN 20 ug in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ACETIC ACID (UNII: Q40Q9N063P) ALANINE (UNII: OF5P57N2ZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65044-9985-2 1.2 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103883 02/22/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103883 02/22/1982 Labeler - Jubilant HollisterStier LLC (069263643) Registrant - Jubilant HollisterStier LLC (069263643)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.