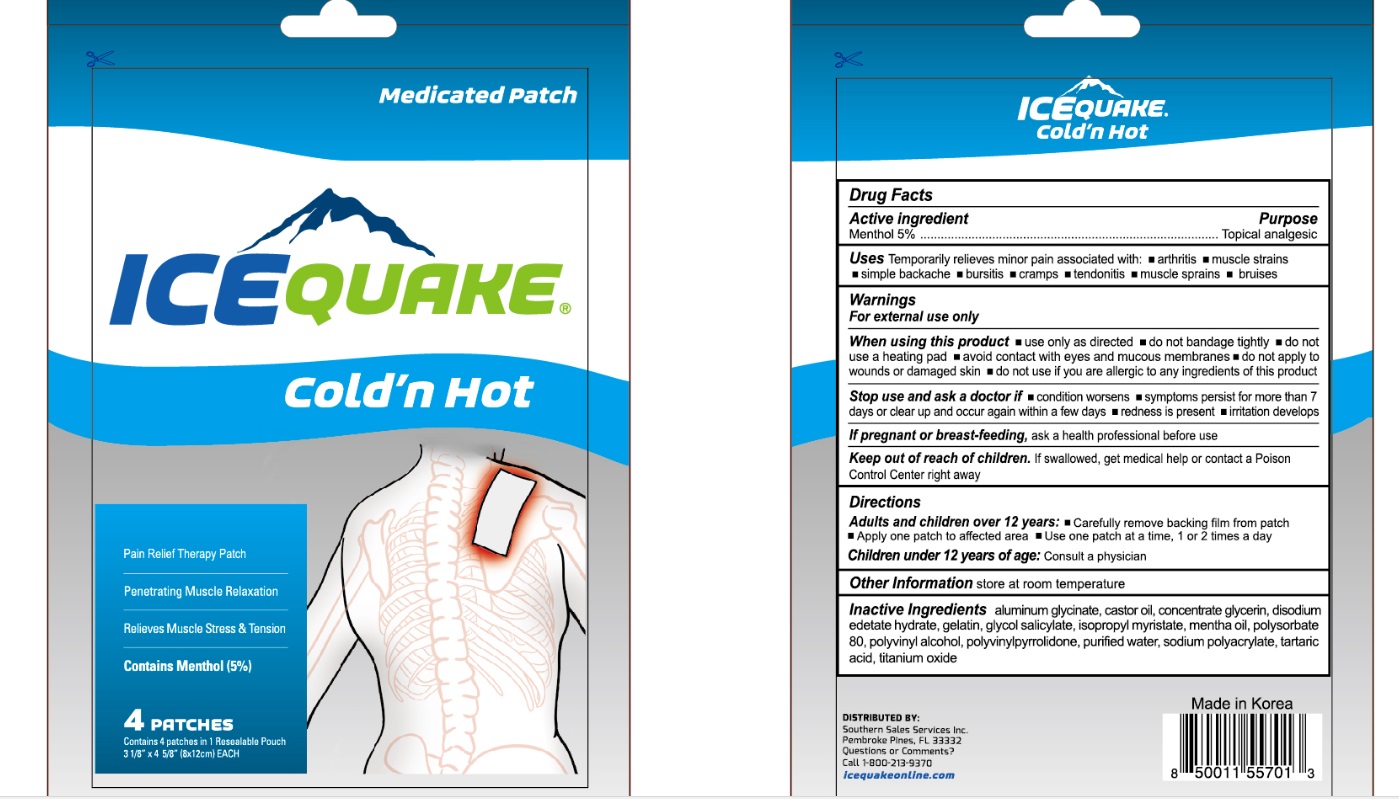
IceQuake Coldn Hot Medicated Patch
IceQuake Coldn Hot Medicated by
Drug Labeling and Warnings
IceQuake Coldn Hot Medicated by is a Otc medication manufactured, distributed, or labeled by Southern Sales & Service, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ICEQUAKE COLDN HOT MEDICATED- menthol patch
Southern Sales & Service, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
IceQuake Coldn Hot Medicated Patch
Drug Facts
Uses
Temporarily relieves minor pain associated with:
- arthritis
- muscle strains
- simple backache
- bursitis
- cramps
- tendonitis
- muscle srains
- bruises
Warnings
For external use only
When using this product
- use only as directed
- do not bandage tightly
- do not use a heating pad
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged skin
- do not use if you are allergic to any ingredients of this product
| ICEQUAKE COLDN HOT MEDICATED
menthol patch |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Southern Sales & Service, Inc. (013114906) |
| Registrant - Southern Sales & Service, Inc. (013114906) |
Revised: 7/2023
Document Id: 00383aa5-130c-f75f-e063-6294a90ab305
Set id: e08b7fb5-260f-42a4-97ad-2083b9f440a0
Version: 3
Effective Time: 20230711