Cetaphil Eczema Soothing Moisturizer by Galderma Laboratories, L.P. / G Production Inc. Drug Facts
Cetaphil Eczema Soothing Moisturizer by
Drug Labeling and Warnings
Cetaphil Eczema Soothing Moisturizer by is a Otc medication manufactured, distributed, or labeled by Galderma Laboratories, L.P., G Production Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CETAPHIL ECZEMA SOOTHING MOISTURIZER- colloidal oatmeal lotion
Galderma Laboratories, L.P.
----------
Drug Facts
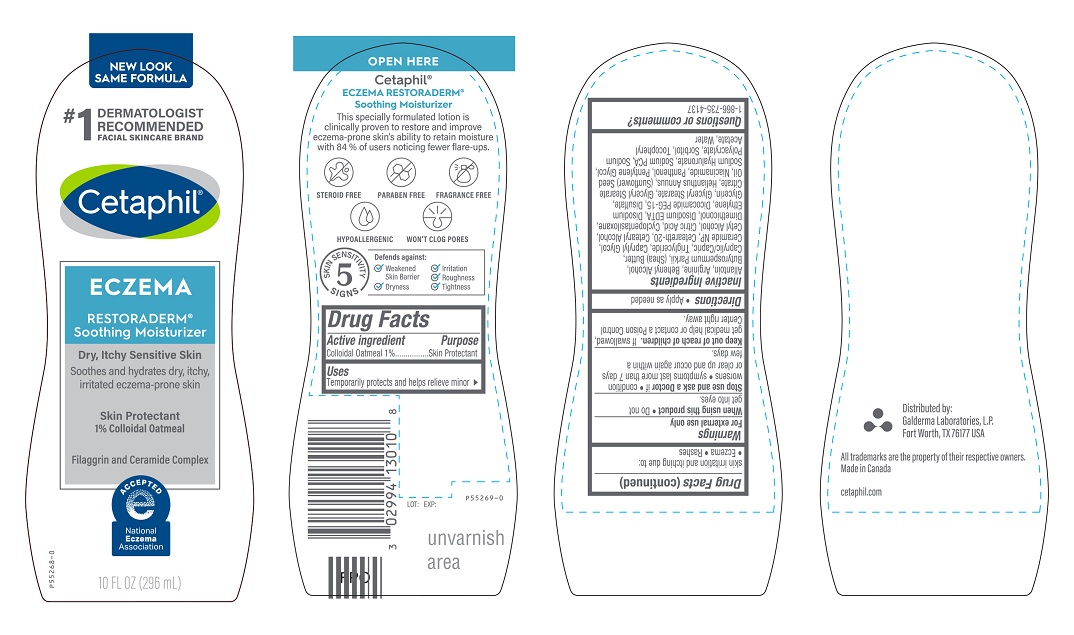
Uses
Temporarily protects and helps relieve minor skin irritation and itching due to:
●Eczema ●Rashes
Warnings
For external use only.
When using this product ● Do not get into eyes.
Stop use and ask a Doctor if ● condition worsens ● symptoms last more than 7 days or clear up and occur again within a few days.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
Allantoin, Arginine, Behenyl Alcohol, Butyrospermum Parkii, (Shea) Butter, Caprylic/Capric, Triglyceride, Caprylyl Glycol, Ceramide NP, Ceteareth-20, Cetearyl Alcohol, Cetyl Alcohol, Citric Acid, Cyclopentasiloxane, Dimethiconol, Disodium EDTA, Disodium Ethylene, Dicocamide PEG-15, Disulfate, Glycerin, Glyceryl Stearate, Glyceryl Stearate Citrate, Helianthus Annuus, (Sunflower) Seed Oil, Niacinamide, Panthenol, Pentylene Glycol, Sodium Hyaluronate, Sodium PCA, Sodium Polyacrylate, Sorbitol, Tocopheryl Acetate, Water
Questions or comments?
1-866-735-4137
Distributed By:
Galderma Laboratories, L.P.
Fort Worth, TX 76177
All trademarks are the property of their respective owners
Made in Canada
Cetaphil.com
PRINCIPLE DISPLAY PANEL - 10 FL OZ bottle
NEW LOOK
SAME FORMULA
#1 Dermatologist Recommended
Facial Skincare Brand
Cetaphil®
ECZEMA
RESTORADERM®
Soothing Moisturizer
Dry, Itchy Senstitive Skin
Soothes and hydrates dry, itchy,
irritated eczema-prone skin
Skin Protectant
1% Colloidal Oatmeal
Filaggrin and Ceramide Complex
National Eczema Association logo
10 FL OZ (296 mL)
| CETAPHIL ECZEMA SOOTHING MOISTURIZER
colloidal oatmeal lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Galderma Laboratories, L.P. (047350186) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| G Production Inc. | 251676961 | manufacture(0299-4124) | |