IPRATROPIUM BROMIDE AND ALBUTEROL SULFATE solution
IPRATROPIUM BROMIDE AND ALBUTEROL SULFATE by
Drug Labeling and Warnings
IPRATROPIUM BROMIDE AND ALBUTEROL SULFATE by is a Prescription medication manufactured, distributed, or labeled by Ritedose Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
The active components in Ipratropium Bromide and Albuterol Sulfate Inhalation Solution are albuterol sulfate and ipratropium bromide.
Albuterol sulfate, is a salt of racemic albuterol and a relatively selective β 2-adrenergic bronchodilator chemically described as α 1-[( tert-butylamino)methyl]-4-hydroxy- m-xylene-α, α'-diol sulfate (2:1) (salt). It has a molecular weight of 576.7 and the empirical formula is (C 13H 21NO 3) 2H 2SO 4. It is a white crystalline powder, soluble in water and slightly soluble in ethanol. The World Health Organization recommended name for albuterol base is salbutamol.
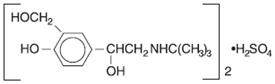
Figure 3.1-1. Chemical structure of albuterol sulfate.
Ipratropium bromide is an anticholinergic bronchodilator chemically described as 8-azoniabicyclo [3.2.1]-octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8methyl-8-(1-methylethyl)-, bromide, monohydrate ( endo, syn)-, (±)-; a synthetic quaternary ammonium compound, chemically related to atropine. It has a molecular weight of 430.4 and the empirical formula is C 20H 30BrNO 3H 2O. It is a white crystalline substance, freely soluble in water and lower alcohols, and insoluble in lipophilic solvents such as ether, chloroform, and fluorocarbons.
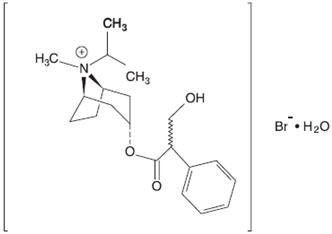
Figure 3.1-2. Chemical structure of ipratropium bromide.
Each 3 mL Sterile Unit-dose Vial contains 0.5 mg of ipratropium bromide (0.017%) and 3 mg 1 albuterol sulfate (0.083%) in an isotonic, sterile, aqueous solution containing sodium chloride and 1 N hydrochloric acid to adjust to pH 4.
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is a clear, colorless solution. It does not require dilution prior to administration by nebulization. For Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, like all other nebulized treatments, the amount delivered to the lungs will depend on patient factors, the jet nebulizer utilized, and compressor performance. Using the Pari-LC-Plus™ nebulizer (with face mask or mouthpiece) connected to a PRONEB™ compressor system, under in vitro conditions, the mean delivered dose from the mouth piece (% nominal dose) was approximately 46% of albuterol and 42% of ipratropium bromide at a mean flow rate of 3.6 L/min. The mean nebulization time was 15 minutes or less. Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should be administered from jet nebulizers at adequate flow rates, via face masks or mouthpieces (see DOSAGE AND ADMINISTRATION).
- 1 Equivalent to 2.5 mg albuterol base
-
CLINICAL PHARMACOLOGY
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is a combination of the β 2-adrenergic bronchodilator, albuterol sulfate, and the anticholinergic bronchodilator, ipratropium bromide.
Albuterol sulfate
Mechanism of Action
The prime action of β-adrenergic drugs is to stimulate adenyl cyclase, the enzyme that catalyzes the formation of cyclic-3',5'-adenosine monophosphate (cAMP) from adenosine triphosphate (ATP). The cAMP thus formed mediates the cellular responses. In vitro studies and in vivo pharmacologic studies have demonstrated that albuterol has a preferential effect on β 2-adrenergic receptors compared with isoproterenol. While it is recognized that β 2-adrenergic receptors are the predominant receptors in bronchial smooth muscle, recent data indicated that 10% to 50% of the β-receptors in the human heart may be β 2-receptors. The precise function of these receptors, however, is not yet established. Albuterol has been shown in most controlled clinical trials to have more effect on the respiratory tract, in the form of bronchial smooth muscle relaxation, than isoproterenol at comparable doses while producing fewer cardiovascular effects. Controlled clinical studies and other clinical experience have shown that inhaled albuterol, like other β-adrenergic agonist drugs, can produce a significant cardiovascular effect in some patients.
Pharmacokinetics
Albuterol sulfate is longer acting than isoproterenol in most patients by any route of administration, because it is not a substrate for the cellular uptake processes for catecholamine nor for the metabolism of catechol-O-methyl transferase. Instead the drug is conjugatively metabolized to albuterol 4'- O-sulfate.
Animal Pharmacology/Toxicology
Intravenous studies in rats with albuterol sulfate have demonstrated that albuterol crosses the blood-brain barrier and reaches brain concentrations amounting to approximately 5% of plasma concentrations. In structures outside of the blood-brain barrier (pineal and pituitary glands), albuterol concentrations were found to be 100 times those found in whole brain.
Studies in laboratory animals (minipigs, rodents, and dogs) have demonstrated the occurrence of cardiac arrhythmias and sudden death (with histological evidence of myocardial necrosis) when beta-agonists and methyl-xanthines are administered concurrently. The clinical significance of these findings is unknown.
Ipratropium bromide
Mechanism of Action
Ipratropium bromide is an anticholinergic (parasympatholytic) agent, which blocks the muscarinic receptors of acetylcholine, and, based on animal studies, appears to inhibit vagally mediated reflexes by antagonizing the action of acetylcholine, the transmitter agent released from the vagus nerve. Anticholinergics prevent the increases in intracellular concentration of cyclic guanosine monophosphate (cGMP), resulting from the interaction of acetylcholine with the muscarinic receptors of bronchial smooth muscle.
Pharmacokinetics
The bronchodilation following inhalation of ipratropium is primarily a local, site-specific effect, not a systemic one. Much of an inhaled dose is swallowed as shown by fecal excretion studies. Following nebulization of a 1-mg dose to healthy volunteers, a mean of 4% of the dose was excreted unchanged in the urine.
Ipratropium bromide is minimally (0% to 9% in vitro) bound to plasma albumin and α 1-acid glycoproteins. It is partially metabolized to inactive ester hydrolysis products. Following intravenous administration, approximately one-half is excreted unchanged in the urine. The half-life of elimination is about 1.6 hours after intravenous administration. Ipratropium bromide that reaches the systemic circulation is reportedly removed by the kidneys rapidly at a rate that exceeds the glomerular filtration rate. The pharmacokinetics of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution or ipratropium bromide have not been studied in the elderly and in patients with hepatic or renal insufficiency (see PRECAUTIONS).
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution
Mechanism of Action
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is expected to maximize the response to treatment in patients with chronic obstructive pulmonary disease (COPD) by reducing bronchospasm through two distinctly different mechanisms: sympathomimetic (albuterol sulfate) and anticholinergic/parasympatholytic (ipratropium bromide). Simultaneous administration of both an anticholinergic and a β2-sympathomimetic is designed to produce greater bronchodilation effects than when either drug is utilized alone at its recommended dosage.
Animal Pharmacology/Toxicology
In 30-day studies in Sprague-Dawley rats and Beagle dogs, subcutaneous doses of up to 205.5 mcg/kg of ipratropium administered with up to 1000 mcg/kg albuterol in rats and 3.16 mcg/kg ipratropium and 15 mcg/kg albuterol in dogs (less than the maximum recommended daily inhalation dose for adults on a mg/m 2 basis) did not cause death or potentiation of the cardiotoxicity induced by albuterol administered alone.
Pharmacokinetics
In a double blind, double period, crossover study, 15 male and female subjects were administered single doses of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution or albuterol sulfate inhalation solution at two times the recommended single doses as two inhalations separated by 15 minutes. The total nebulized dose of albuterol sulfate from both treatments was 6 mg and the total dose of ipratropium bromide from Ipratropium Bromide and Albuterol Sulfate Inhalation Solution was 1 mg. Peak albuterol plasma concentrations occurred at 0.8 hours after dosing for both treatments. The mean peak albuterol concentration following administration of albuterol sulfate alone was 4.86 (± 2.65) mg/mL and it was 4.65 (± 2.92) mg/mL for Ipratropium Bromide and Albuterol Sulfate Inhalation Solution. Mean AUC values for the two treatments were 26.6 (± 15.2) ng∙hr/mL (albuterol sulfate alone) versus 24.2 (± 14.5) ng∙hr/mL (Ipratropium Bromide and Albuterol Sulfate Inhalation Solution). The mean t 1/2 values were 7.2 (± 1.3) hours (albuterol sulfate alone) and 6.7 (± 1.7) hours (Ipratropium Bromide and Albuterol Sulfate Inhalation Solution). A mean of 8.4 (± 8.9)% of the albuterol dose was excreted unchanged in urine following administration of two vials of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution which is similar to 8.8 (± 7.3)% that was obtained from albuterol sulfate inhalation solution. There were no statistically significant differences in the pharmacokinetics of albuterol between the two treatments. For ipratropium, a mean of 3.9 (± 5.1)% of the ipratropium bromide dose was excreted unchanged in urine following two vials of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, which is comparable with previously reported data.
Clinical Trials
In a 12 week, randomized, double-blind, positive-control, crossover study of albuterol sulfate, ipratropium bromide, and Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, 863 COPD patients were evaluated for bronchodilator efficacy comparing ipratropium bromide and albuterol sulfate with albuterol sulfate and ipratropium bromide alone.
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution demonstrated significantly better changes in FEV 1, as measured from baseline to peak response, when compared with either albuterol sulfate or ipratropium bromide. Ipratropium Bromide and Albuterol Sulfate Inhalation Solution was also shown to have the rapid onset associated with albuterol sulfate, with a mean time to peak FEV 1 of 1.5 hours, and the extended duration associated with ipratropium bromide with a duration of 15% response in FEV 1 of 4.3 hours.
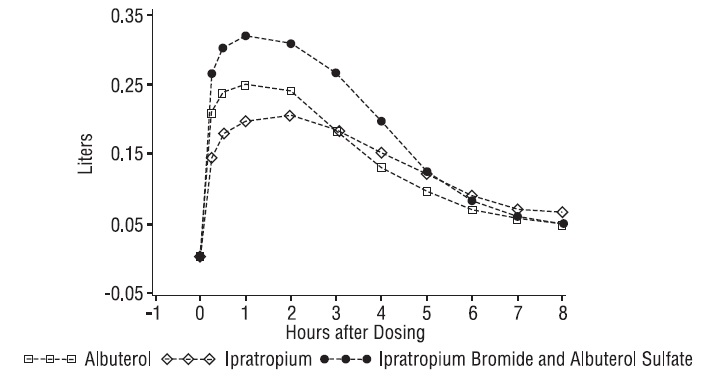
Figure 3. 1-3. Mean Change in FEV 1 - Measured on Day 14
This study demonstrated that each component of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution contributed to the improvement in pulmonary function, especially during the first 4 to 5 hours after dosing, and that Ipratropium Bromide and Albuterol Sulfate Inhalation Solution was significantly more effective than albuterol sulfate or ipratropium bromide alone.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Paradoxical Bronchospasm
In the clinical study of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, paradoxical bronchospasm was not observed. However, paradoxical bronchospasm has been observed with both inhaled ipratropium bromide and albuterol products and can be life-threatening. If this occurs, Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should be discontinued immediately and alternative therapy instituted.
Do Not Exceed Recommended Dose
Fatalities have been reported in association with excessive use of inhaled products containing sympathomimetic amines and with the home use of nebulizers.
Cardiovascular Effect
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, like other beta adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon for Ipratropium Bromide and Albuterol Sulfate Inhalation Solution at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta agonists have been reported to produce ECG changes, such as flattening of the T-wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, like other sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions to albuterol and/or ipratropium bromide may occur after the administration of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution as demonstrated by rare cases of urticaria, angioedema, rash, pruritus, oropharyngeal edema, bronchospasm, and anaphylaxis.
-
PRECAUTIONS
General
1. Effects Seen with Sympathomimetic Drugs
As with all products containing sympathomimetic amines, Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension; in patients with convulsive disorders, hyperthyroidism, or diabetes mellitus; and in patients who are unusually responsive to sympathomimetic amines. Large doses of intravenous albuterol have been reported to aggravate pre-existing diabetes mellitus and ketoacidosis. Additionally, β-agonists may cause a decrease in serum potassium in some patients, possibly through intracellular shunting. The decrease is usually transient, not requiring supplementation.
Information for Patients
The action of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should last up to 5 hours. Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should not be used more frequently than recommended. Patients should be instructed not to increase the dose or frequency of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution without consulting their healthcare provider. If symptoms worsen, patients should be instructed to seek medical consultation.
Patients must avoid exposing their eyes to this product as temporary pupillary dilation, blurred vision, eye pain, or precipitation or worsening of narrow-angle glaucoma may occur, and therefore proper nebulizer technique should be assured, particularly if a mask is used.
If a patient becomes pregnant or begins nursing while on Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, they should contact their healthcare provider about use of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution.
See the illustrated Patient's Instruction for Use in the product package insert.
Drug Interactions
Anticholinergic agents
Although ipratropium bromide is minimally absorbed into the systemic circulation, there is some potential for an additive interaction with concomitantly used anticholinergic medications. Caution is, therefore, advised in the co-administration of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution with other drugs having anticholinergic properties.
β-adrenergic agents
Caution is advised in the co-administration of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution and other sympathomimetic agents due to the increased risk of adverse cardiovascular effects.
β-receptor blocking agents
These agents and albuterol sulfate inhibit the effect of each other. β-receptor blocking agents should be used with caution in patients with hyperreactive airways, and if used, relatively selective β 1 selective agents are recommended.
Diuretics
The electrocardiogram (ECG) changes and/or hypokalemia that may result from the administration of non-potassium sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by β-agonists, especially when the recommended dose of the β-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the co-administration of β-agonist-containing drugs, such as Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, with non-potassium sparing diuretics.
Monoamine oxidase inhibitors or tricyclic antidepressants
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents because the action of albuterol sulfate on the cardiovascular system may be potentiated.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Albuterol Sulfate
In a 2-year study in Sprague-Dawley rats, albuterol sulfate caused a significant dose-related increase in the incidence of benign leiomyomas of the mesovarium at and above dietary doses of 2 mg/kg (approximately equal to the maximum recommended daily inhalation dose for adults on a mg/m 2 basis). In another study, this effect was blocked by the coadministration of propranolol, a non-selective beta-adrenergic antagonist.
In an 18-month study in CD-1 mice, albuterol sulfate showed no evidence of tumorigenicity at dietary doses up to 500 mg/kg (approximately 140 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis). In a 22-month study in Golden hamsters, albuterol sulfate showed no evidence of tumorigenicity at dietary doses up to 50 mg/kg (approximately 20 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis).
Albuterol sulfate was not mutagenic in the Ames test or a mutation test in yeast. Albuterol sulfate was not clastogenic in a human peripheral lymphocyte assay or in an AH1 strain mouse micronucleous assay.
Reproduction studies in rats demonstrated no evidence of impaired fertility at oral doses of albuterol sulfate up to 50 mg/kg (approximately 25 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis).
Ipratropium bromide
In 2-year studies in Sprague-Dawley rats and CD-1 mice, ipratropium bromide showed no evidence of tumorigenicity at oral doses up to 6 mg/kg (approximately 15 times and 8 times the maximum recommended daily inhalation dose for adults in rats and mice respectively, on a mg/m 2 basis).
Ipratropium bromide was not mutagenic in the Ames test and mouse dominant lethal test. Ipratropium bromide was not clastogenic in a mouse micronucleous assay.
A reproduction study in rats demonstrated decreased conception and increased resorptions when ipratropium bromide was administered orally at a dose of 90 mg/kg (approximately 240 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis). These effects were not seen with a dose of 50 mg/kg (approximately 140 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis).
Pregnancy
TERATOGENIC EFFECTS
Pregnancy Category C
Albuterol sulfate
Pregnancy Category C
Albuterol sulfate has been shown to be teratogenic in mice. A study in CD-1 mice given albuterol sulfate subcutaneously showed cleft palate formation in 5 of 111 (4.5%) fetuses at 0.25 mg/kg (less than the maximum recommended daily inhalation dose for adults on a mg/m 2 basis) and in 10 of 108 (9.3%) fetuses at 2.5 mg/kg (approximately equal to the maximum recommended daily inhalation dose for adults on a mg/m 2 basis). The drug did not induce cleft palate formation when administered subcutaneously at a dose of 0.025 mg/kg (less than the maximum recommended daily inhalation dose for adults on a mg/m 2 basis). Cleft palate formation also occurred in 22 of 72 (30.5%) fetuses from females treated subcutaneously with 2.5 mg/kg isoproterenol (positive control).
A reproduction study in Stride rabbits revealed cranioschisis in 7 of 19 (37%) fetuses when albuterol was administered orally at a dose of 50 mg/kg (approximately 55 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis).
A study in which pregnant rats were dosed with radiolabeled albuterol sulfate demonstrated that drug-related material is transferred from the maternal circulation to the fetus.
During worldwide marketing experience, various congenital anomalies, including cleft palate and limb defects, have been reported in the offspring of patients being treated with albuterol. Some of the mothers were taking multiple medications during their pregnancies. Because no consistent pattern of defects can be discerned, a relationship between albuterol use and congenital anomalies has not been established.
Ipratropium bromide
Pregnancy Category B
Reproduction studies in CD-1 mice, Sprague-Dawley rats and New Zealand rabbits demonstrated no evidence of teratogenicity at oral doses up to 10, 100, and 125 mg/kg, respectively (approximately 15, 270, and 680 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis). Reproduction studies in rats and rabbits demonstrated no evidence of teratogenicity at inhalation doses up to 1.5 and 1.8 mg/kg, respectively (approximately 4 and 10 times the maximum recommended daily inhalation dose for adults on a mg/m 2 basis). There are no adequate and well-controlled studies of the use of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, albuterol sulfate, or ipratropium bromide in pregnant women. Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery
Oral albuterol sulfate has been shown to delay preterm labor in some reports. Because of the potential of albuterol to interfere with uterine contractility, use of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution during labor should be restricted to those patients in whom the benefits clearly outweigh the risks.
Nursing Mothers
It is not known whether the components of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution are excreted in human milk. Although lipid-insoluble quaternary bases pass into breast milk, it is unlikely that ipratropium bromide would reach the infant to an important extent, especially when taken as a nebulized solution. Because of the potential for tumorigenicity shown for albuterol sulfate in some animals, a decision should be made whether to discontinue nursing or discontinue Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution in patients below 18 years of age have not been established.
Geriatric Use
Of the total number of subjects in clinical studies of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution, 62 percent were 65 and over, while 19 percent were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
ADVERSE REACTIONS
Adverse reaction information concerning Ipratropium Bromide and Albuterol Sulfate Inhalation Solution was derived from the 12-week controlled clinical trial.
ADVERSE EVENTS OCCURRING IN ≥ 1% OF ≥ 1 TREATMENT GROUP(S) AND WHERE THE COMBINATION TREATMENT SHOWED THE HIGHEST PERCENTAGE Body System
COSTART TermAlbuterol
n (%)Ipratropium
n (%)Ipratropium and Albuterol
n (%)NUMBER OF PATIENTS 761 754 765 N (%) Patients with AE 327 (43.0) 329 (43.6) 367 (48.0) BODY AS A WHOLE Pain 8 (1.1) 4 (0.5) 10 (1.3) Pain chest 11 (1.4) 14 (1.9) 20 (2.6) DIGESTIVE Diarrhea 5 (0.7) 9 (1.2) 14 (1.8) Dyspepsia 7 (0.9) 8 (1.1) 10 (1.3) Nausea 7 (0.9) 6 (0.8) 11 (1.4) MUSCULO-SKELETAL Cramps leg 8 (1.1) 6 (0.8) 11 (1.4) RESPIRATORY Bronchitis 11 (1.4) 13 (1.7) 13 (1.7) Lung Disease 36 (4.7) 34 (4.5) 49 (6.4) Pharyngitis 27 (3.5) 27 (3.6) 34 (4.4) Pneumonia 7 (0.9) 8 (1.1) 10 (1.3) UROGENITAL Infection urinary tract 3 (0.4) 9 (1.2) 12 (1.6) Additional adverse reactions reported in more than 1% of patients treated with Ipratropium Bromide and Albuterol Sulfate Inhalation Solution included constipation and voice alterations.
In the clinical trial, there was a 0.3% incidence of possible allergic-type reactions, including skin rash, pruritus, and urticaria.
Additional information derived from the published literature on the use of albuterol sulfate and ipratropium bromide singly or in combination includes precipitation or worsening of narrow-angle glaucoma, acute eye pain, blurred vision, mydriasis, paradoxical bronchospasm, wheezing, exacerbation of COPD symptoms, drowsiness, aching, flushing, upper respiratory tract infection, palpitations, taste perversion, elevated heart rate, sinusitis, back pain, sore throat and metabolic acidosis. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
OVERDOSAGE
The effects of overdosage with Ipratropium Bromide and Albuterol Sulfate Inhalation Solution are expected to be related primarily to albuterol sulfate, since ipratropium bromide is not well-absorbed systemically after oral or aerosol administration. The expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of symptoms such as seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats per minute, arrhythmia, nervousness, headache, tremor, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, insomnia, and exaggeration of pharmacological effects listed in ADVERSE REACTIONS. Hypokalemia may also occur. As with all sympathomimetic aerosol medications, cardiac arrest and even death may be associated with abuse of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution. Treatment consists of discontinuation of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution.
The oral median lethal dose of albuterol sulfate in mice is greater than 2000 mg/kg (approximately 540 times the maximum recommended daily inhalation dose of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution on a mg/m 2 basis). The subcutaneous median lethal dose of albuterol sulfate in mature rats and small young rats is approximately 450 and 2000 mg/kg respectively (approximately 240 and 1100 times the maximum recommended daily inhalation dose of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution on a mg/m 2 basis, respectively). The inhalation median lethal dose has not been determined in animals. The oral median lethal dose of ipratropium bromide in mice, rats and dogs is greater than 1000 mg/kg, approximately 1700 mg/kg and approximately 400 mg/kg, respectively (approximately 1400, 4600, and 3600 times the maximum recommended daily inhalation dose in adults on a mg/m 2 basis, respectively).
-
DOSAGE AND ADMINISTRATION
The recommended dose of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is one 3 mL vial administered 4 times per day via nebulization with up to 2 additional 3 mL doses allowed per day, if needed. Safety and efficacy of additional doses or increased frequency of administration of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution beyond these guidelines has not been studied and the safety and efficacy of extra doses of albuterol sulfate or ipratropium bromide in addition to the recommended doses of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution have not been studied.
The use of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution can be continued as medically indicated to control recurring bouts of bronchospasm. If a previously effective regimen fails to provide the usual relief, medical advice should be sought immediately, as this is often a sign of worsening COPD, which would require reassessment of therapy.
A Pari-LC-Plus™ nebulizer (with face mask or mouthpiece) connected to a PRONEB™ compressor was used to deliver Ipratropium Bromide and Albuterol Sulfate Inhalation Solution to each patient in one U.S. clinical study. The safety and efficacy of Ipratropium Bromide and Albuterol Sulfate Inhalation Solution delivered by other nebulizers and compressors have not been established.
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should be administered via jet nebulizer connected to an air compressor with an adequate air flow, equipped with a mouthpiece or suitable face mask.
-
HOW SUPPLIED
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is supplied as a 3-mL sterile solution for nebulization in sterile low-density polyethylene unit-dose vials. Store in pouch until time of use. Supplied in cartons as listed below.
NDC: 76204-600-01 30 vials per carton / 1 vial per foil pouch NDC: 76204-600-05 30 vials per carton / 5 vials per foil pouch NDC: 76204-600-12 60 vials per carton / 5 vials per foil pouch NDC: 76204-600-30 30 vials per carton / 30 vials per foil pouch NDC: 76204-600-60 60 vials per carton / 30 vials per foil pouch -
PATIENT PACKAGE INSERT
Ipratropium Bromide and Albuterol Sulfate
Inhalation Solution
0.5 mg / 3 mg per 3 mLPatient's Instructions for Use
Read this patient information completely every time your prescription is filled as information may have changed. Keep these instructions with your medication as you may want to read them again.
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution should only be used under the direction of a physician. Your physician and pharmacist have more information about Ipratropium Bromide and Albuterol Sulfate Inhalation Solution and the condition for which it has been prescribed. Contact them if you have additional questions.
Storing your Medicine
Store Ipratropium Bromide and Albuterol Sulfate Inhalation Solution between 2°C and 25°C (36°F and 77°F). Vials should be protected from light before use, therefore, keep unused vials in the foil pouch or carton. Do not use after the expiration (EXP) date printed on the carton.
Dose
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution is supplied as a single-dose, ready-to-use vial containing 3 mL of solution. No mixing or dilution is needed. Use one new vial for each nebulizer treatment.
FOLLOW THESE DIRECTIONS FOR USE OF YOUR NEBULIZER/COMPRESSOR OR THE DIRECTIONS GIVEN BY YOUR HEALTHCARE PROVIDER. A TYPICAL EXAMPLE IS SHOWN BELOW.
Instructions for Use
- Remove one vial from the foil pouch. Place remaining vials back into pouch for storage.
- Twist the cap completely off the vial and squeeze the contents into the nebulizer reservoir (Figure 1).
- Connect the nebulizer to the mouthpiece or face mask (Figure 2).
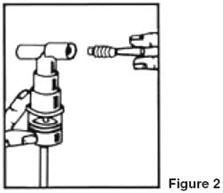
- Connect the nebulizer to the compressor.
- Sit in a comfortable, upright position; place the mouthpiece in your mouth (Figure 3) or put on the face mask (Figure 4); and turn on the compressor.
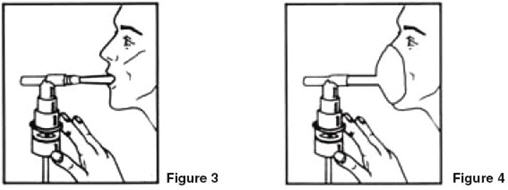
- Breathe as calmly, deeply and evenly as possible through your mouth until no more mist is formed in the nebulizer chamber (about 5-15 minutes). At this point, the treatment is finished.
- Clean the nebulizer (see manufacturer's instructions).
Manufactured by:
The Ritedose Corporation
Columbia, SC 29203 for
Ritedose Pharmaceuticals, LLC
Columbia, SC 29203DEC 2013
RPIN0053

-
Patient Information
Ipratropium Bromide and Albuterol Sulfate Inhalation Solution
0.5 mg / 3 mg per 3 mLPrescription Only.
Read the patient information that comes with Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What is Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg is a combination of two medicines called bronchodilators. Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg contains albuterol sulfate, which is a beta-adrenergic agonist, and ipratropium bromide, which is an anticholinergic. These two medicines work together to help open the airways in your lungs. Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg is used to help treat airway narrowing (bronchospasm) that happens with chronic obstructive pulmonary disease (COPD) in adult patients who need to use more than one bronchodilator medicine.
Who should not use Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Do not use Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg if you: Are allergic to any of the ingredients in Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg or to atropine. The active ingredients are albuterol sulfate and ipratropium bromide. See the end of this leaflet for a complete list of ingredients in Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg.
Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg has not been studied in patients younger than 18 years of age.
What should I tell my doctor before I start using Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Tell your doctor about all of your conditions, including if you:
- Have heart problems. This includes coronary artery disease and heart rhythm problems.
- Have high blood pressure
- Have diabetes
- Have or had seizures
- Have a thyroid problem called hyperthyroidism
- Have an eye problem called narrow-angle glaucoma
- Have liver or kidney problems
- Have problems urinating due to bladder-neck blockage or an enlarged prostate (men)
- Are pregnant or planning to become pregnant. It is not known if Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg can harm your unborn baby. You and your doctor will have to decide if Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg is right for you during a pregnancy.
- Are breastfeeding. It is not known if Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg passes into your milk or if it can harm your baby. You and your doctor should decide whether you should take Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg or breastfeed, but not both.
Tell your doctor about all the medicines you take including prescription and non-prescription medicines, vitamins and herbal supplements. Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg and other medicines can interact. This may cause serious side effects. Especially tell your doctor if you take:
- Other medicines that contain anticholinergics such as ipratropium bromide. This also includes medicines used for Parkinson's disease.
- Other medicines that contain beta-agonists such as albuterol sulfate. These are usually used to treat airway narrowing (bronchospasm).
- Medicines called beta-blockers. These are usually used for high blood pressure or heart problems.
- Medicines called "water pills" (diuretics)
- Medicines for depression called monoamine oxidase inhibitors (MAOIs) or tricyclic antidepressants.
Ask your doctor or pharmacist if you are not sure if you take any of these types of medicines. Know the medicines you take. Keep a list of them and show it to your doctor and pharmacists when you get a new medicine.
How should I use Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
- Read the Patient's Instructions for Use that you get with your prescription. Talk to your doctor or pharmacist if you have any questions.
- Take Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg exactly as prescribed by your doctor. Do not change your dose or how often you use Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg without talking to your doctor. Inhale Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg through your mouth and into your lungs using a machine called a nebulizer.
- Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg may help to open your airways for up to 5 hours after taking this medicine. If Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg does not help your airway narrowing (bronchospasm) or your bronchospasm gets worse, call your doctor right away or get emergency help if needed.
What should I avoid while using Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Do not get Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg in your eyes. Be careful not to spray Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg in your eyes while you are using your nebulizer. Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg can cause the following short-term eye problems:
- Enlarged pupils
- Blurry vision
- Eye pain
Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg can cause a serious eye problem called narrow-angle glaucoma or worsen the narrow-angle glaucoma you already have.
What are the possible side effects with Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg may cause the following serious side effects:
- Worsening of the narrowing in your airways (bronchospasm). This side effect can be life-threatening and has happened with both of the medicines that are in Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg. Stop Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg and call your doctor right away or get emergency help if your breathing problems get worse while or after using Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg.
-
Serious and life-threatening allergic reactions. Symptoms of a serious allergic reaction include:
- Hives, rash
- Swelling of your face, eyelids, lips, tongue, or throat, and trouble swallowing
- Worsening of your breathing problems such as wheezing, chest tightness or shortness of breath
- Shock (loss of blood pressure and consciousness)
The most common side effects with Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg include lung disease, sore throat, chest pain, constipation, diarrhea, bronchitis, urinary tract infection, leg cramps, nausea, upset stomach, voice changes, and pain.
These are not all the side effects with Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg. For a complete list, ask your doctor or pharmacist.
How should I store Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
- Store Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg between 36° and 77°F (2° and 25°C). Protect from light. Keep the unused vials in the foil pouch or carton.
- Safely discard Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg that is out-of-date or no longer needed.
- Keep Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg and all medicines out of the reach of children.
General advice about Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg
Medicines are sometimes prescribed for conditions that are not mentioned in the patient information leaflets. Do not use Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg for a condition for which it was not prescribed. Do not give Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg that is written for healthcare professionals.
What are the ingredients in Ipratropium Bromide 0.5 mg and Albuterol Sulfate 3 mg?
Active Ingredients: ipratropium bromide and albuterol sulfate
Inactive Ingredients: sodium chloride and 1 N hydrochloric acid.
Manufactured By:
The Ritedose Corporation
Columbia, SC 29203 for
Ritedose Pharmaceuticals, LLC
Columbia, SC 29203
DEC 2013
RPIN0053
- SPL UNCLASSIFIED SECTION
-
PRNCIPAL DISPLAY PANEL - 30 ct Singles Carton with New Design
NDC: 76204-600-01
Ipratropium Bromide and Albuterol Sulfate
Inhalation Solution0.5 mg / 3 mg per 3 mL
FOR ORAL INHALATION ONLY
STORAGE CONDITIONS: PROTECT FROM LIGHT. Unit-dose vials should remain stored in the protective foil pouch at all times. Once removed from the foil pouch, the individual vials should be used within one week.
Discard if the solution is not colorless.
Store between 2°C and 25°C (36°F and 77°F).KEEP OUT OF REACH OF CHILDREN.
USUAL DOSAGE: See accompanying prescribing information.
USE ONLY AS DIRECTED BY YOUR PHYSICIAN.
DO NOT EXCEED RECOMMENDED DOSAGE.ATTENTION PHARMACIST: Detach "Patient's Instructions For Use" from package insert and dispense with solution.
Rx Only
30 x 3 mL Sterile Unit-Dose Vials
-
PRINCIPAL DISPLAY PANEL - 30 Vial Carton
NDC: 76204-600-30
Ipratropium Bromide and Albuterol Sulfate
Inhalation Solution0.5 mg / 3 mg per 3 mL
FOR ORAL INHALATION ONLY
STORAGE CONDITIONS: PROTECT FROM LIGHT. Unit-dose vials should remain stored
in the protective foil pouch at all times. Once removed from the foil pouch, the individual
vials should be used within one week. Discard if the solution is not colorless.
Store between 2°C and 25°C (36°F and 77°F).USUAL DOSAGE: See accompanying prescribing information.
USE ONLY AS DIRECTED BY YOUR PHYSICIAN.
DO NOT EXCEED RECOMMENDED DOSAGE.ATTENTION PHARMACIST: Detach "Patient's Instructions
For Use" from package insert and dispense with solution.Manufactured by:
The Ritedose Corporation
Columbia, SC 29203 for
Ritedose Pharmaceuticals, LLC
Columbia, SC 29203RITEDOSE
PHARMACEUTICALSRx Only
30 x 3 mL Sterile Unit-Dose Vials (1 pouch of 30 - 3 mL vials)
-
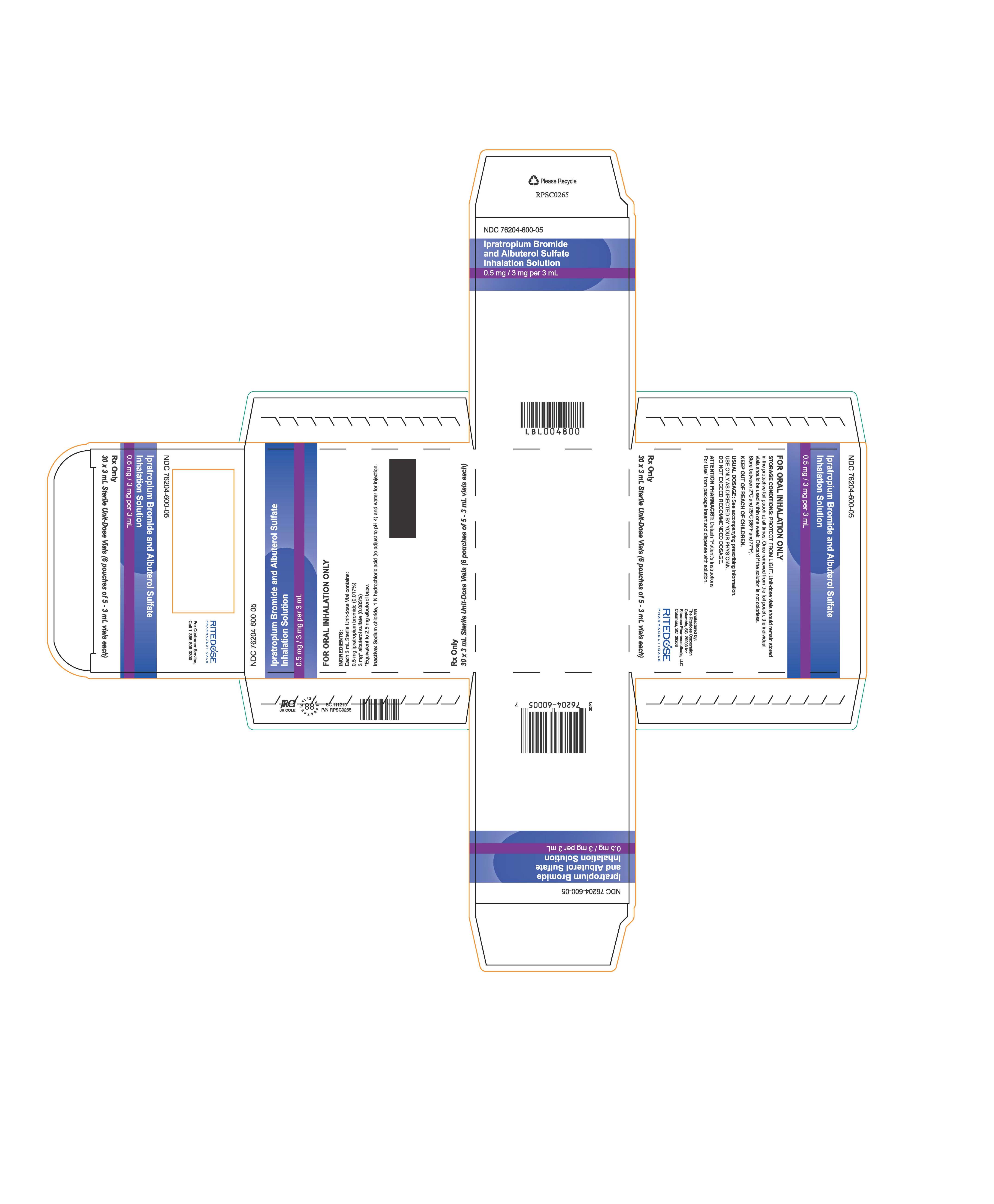
PRINCIPAL DISPLAY PANEL - 30 Cards Carton
NDC: 76204-600-05
Ipratropium Bromide and Albuterol Sulfate
Inhalation Solution0.5 mg / 3 mg per 3 mL
FOR ORAL INHALATION ONLY
STORAGE CONDITIONS: PROTECT FROM LIGHT. Unit-dose vials should remain stored
in the protective foil pouch at all times. Once removed from the foil pouch, the individual
vials should be used within one week. Discard if the solution is not colorless.
Store between 2°C and 25°C (36°F and 77°F).KEEP OUT OF REACH OF CHILDREN.
USUAL DOSAGE: See accompanying prescribing information.
USE ONLY AS DIRECTED BY YOUR PHYSICIAN.
DO NOT EXCEED RECOMMENDED DOSAGE.ATTENTION PHARMACIST: Detach "Patient's Instructions
For Use" from package insert and dispense with solution.Manufactured by:
The Ritedose Corporation
Columbia, SC 29203 for
Ritedose Pharmaceuticals, LLC
Columbia, SC 29203RITEDOSE
PHARMACEUTICALSRx Only
30 x 3 mL Sterile Unit-Dose Vials (6 pouches of 5 - 3 mL vials each)
-
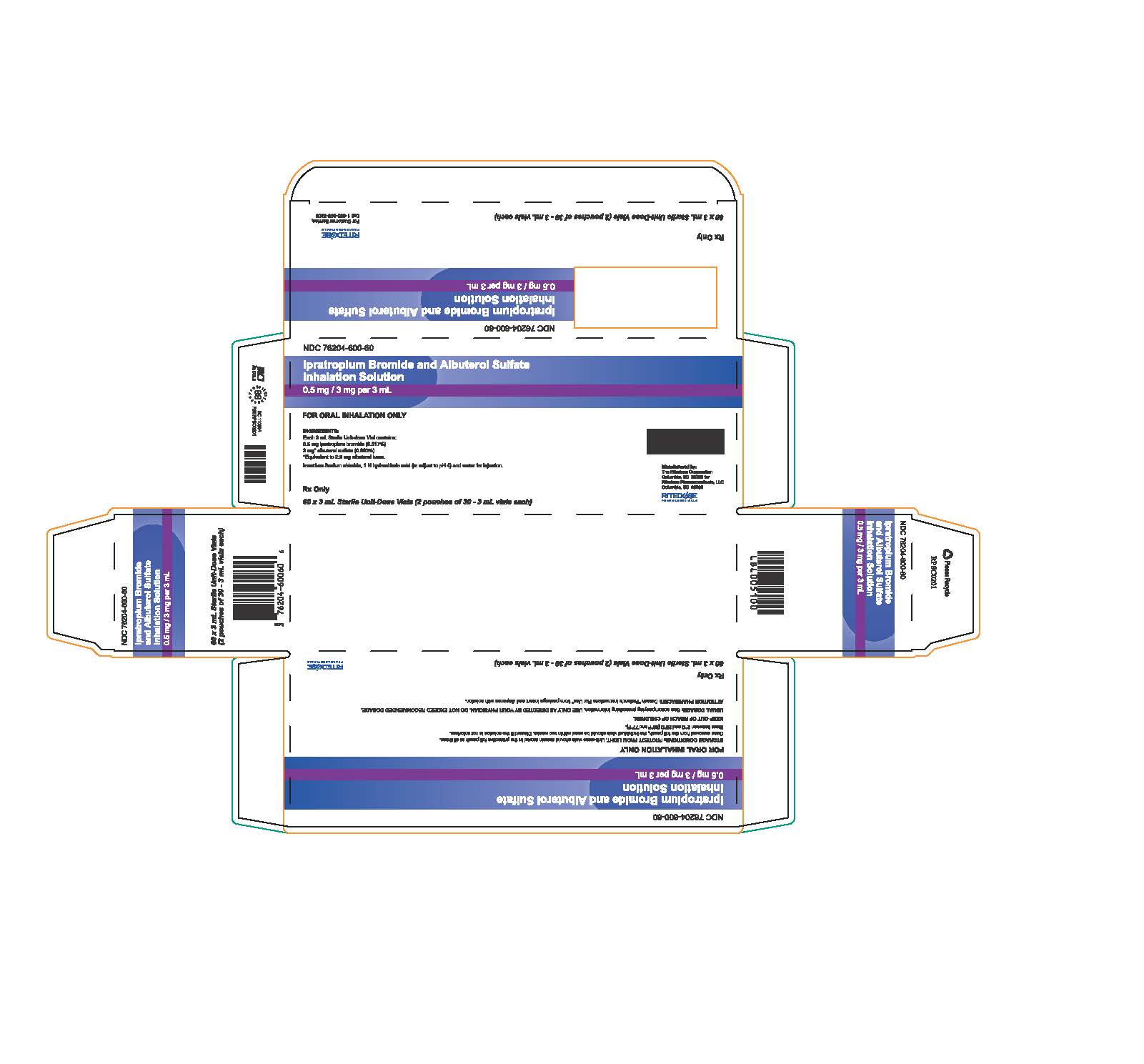
PRINCIPAL DISPLAY PANEL - 60 ct Bricks
NDC: 76204-600-60
Ipratropium Bromide and Albuterol Sulfate
Inhalation Solution0.5 mg / 3 mg per 3 mL
FOR ORAL INHALATION ONLY
STORAGE CONDITIONS: PROTECT FROM LIGHT. Unit-dose vials should remain stored in the protective foil pouch at all times. Once removed from the foil pouch, the individual vials should be used within two weeks. Discard if the solution is not colorless.
Store between 2°C and 25°C (36°F and 77°F).KEEP OUT OF REACH OF CHILDREN.
USUAL DOSAGE: See accompanying prescribing information. USE ONLY AS DIRECTED BY YOUR PHYSICIAN. DO NOT EXCEED RECOMMENDED DOSAGE.
ATTENTION PHARMACIST: Detach "Patient's Instructions For Use" from package insert and dispense with solution.
Rx Only
60 x 3 mL Sterile Unit-Dose Vials (2 pouches of 30 - 3 mL vials each)
-
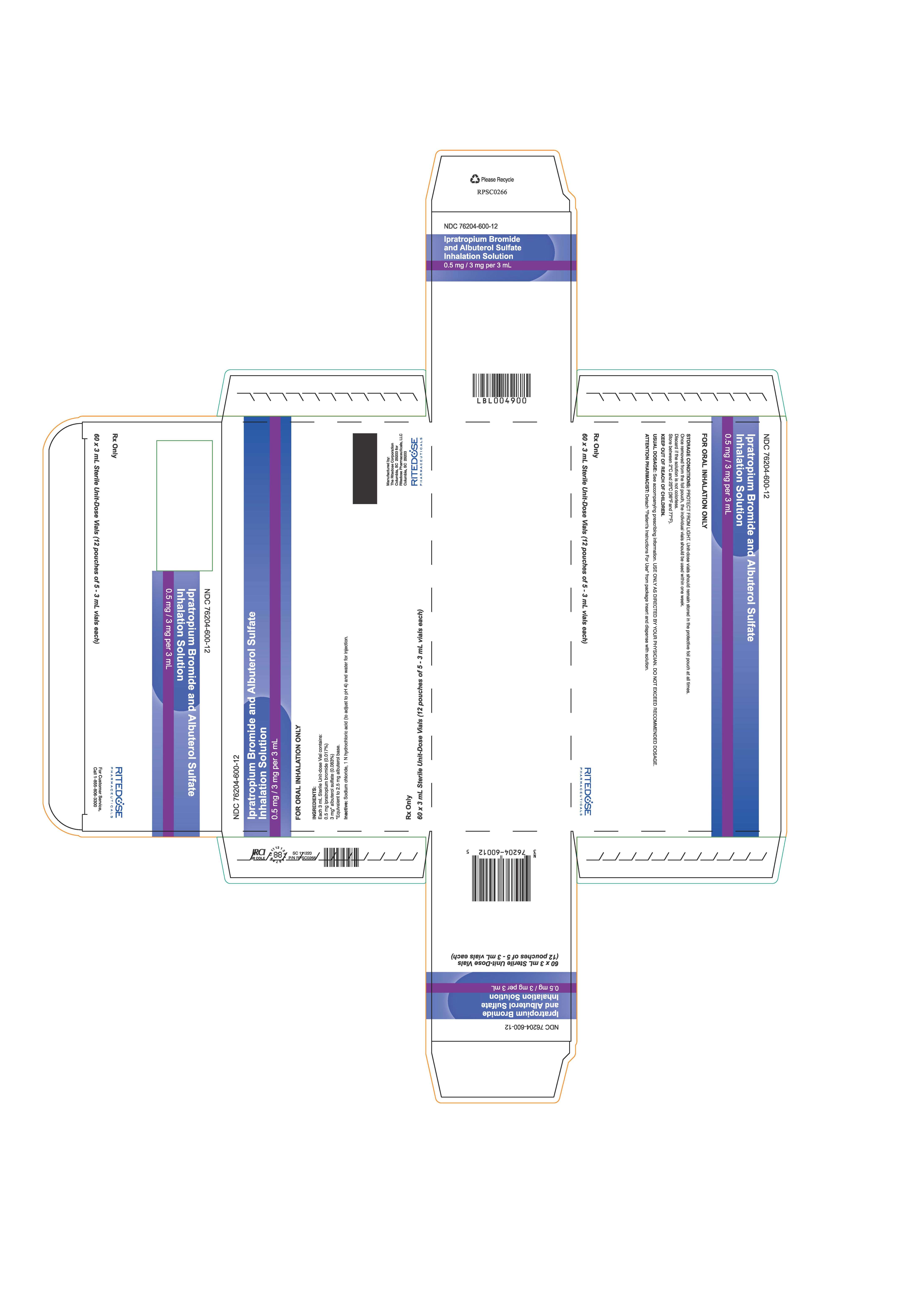
PRINCIPAL DISPLAY PANEL - 60 ct Cards Carton
NDC: 76204-600-12
Ipratropium Bromide and Albuterol Sulfate
Inhalation Solution0.5 mg / 3 mg per 3 mL
FOR ORAL INHALATION ONLY
STORAGE CONDITIONS: PROTECT FROM LIGHT. Unit-dose vials should remain stored in the protective foil pouch at all times. Once removed from the foil pouch, the individual vials should be used within one week.
Discard if the solution is not colorless.
Store between 2°C and 25°C (36°F and 77°F).KEEP OUT OF REACH OF CHILDREN.
USUAL DOSAGE: See accompanying prescribing information. USE ONLY AS DIRECTED BY YOUR PHYSICIAN. DO NOT EXCEED RECOMMENDED DOSAGE.
ATTENTION PHARMACIST: Detach "Patient's Instructions For Use" from package insert and dispense with solution.
Rx Only
60 x 3 mL Sterile Unit-Dose Vials (12 pouches of 5 - 3 mL vials each)
-
INGREDIENTS AND APPEARANCE
IPRATROPIUM BROMIDE AND ALBUTEROL SULFATE
ipratropium bromide and albuterol sulfate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 76204-600 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBUTEROL SULFATE (UNII: 021SEF3731) (ALBUTEROL - UNII:QF8SVZ843E) ALBUTEROL 2.5 mg in 3 mL IPRATROPIUM BROMIDE (UNII: J697UZ2A9J) (IPRATROPIUM - UNII:GR88G0I6UL) IPRATROPIUM BROMIDE ANHYDROUS 0.5 mg in 3 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76204-600-05 6 in 1 CARTON 10/15/2012 1 5 in 1 POUCH 1 3 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 76204-600-01 30 in 1 CARTON 10/15/2012 2 1 in 1 POUCH 2 3 mL in 1 AMPULE; Type 0: Not a Combination Product 3 NDC: 76204-600-12 12 in 1 CARTON 10/15/2012 3 5 in 1 POUCH 3 3 mL in 1 AMPULE; Type 0: Not a Combination Product 4 NDC: 76204-600-30 1 in 1 CARTON 10/15/2012 4 30 in 1 POUCH 4 3 mL in 1 AMPULE; Type 0: Not a Combination Product 5 NDC: 76204-600-60 2 in 1 CARTON 10/15/2012 5 30 in 1 POUCH 5 3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202496 10/15/2012 Labeler - Ritedose Pharmaceuticals, LLC (968062294)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.