Zilactin-B by Applied Laboratories, Inc. Zilactin B Applied
Zilactin-B by
Drug Labeling and Warnings
Zilactin-B by is a Otc medication manufactured, distributed, or labeled by Applied Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
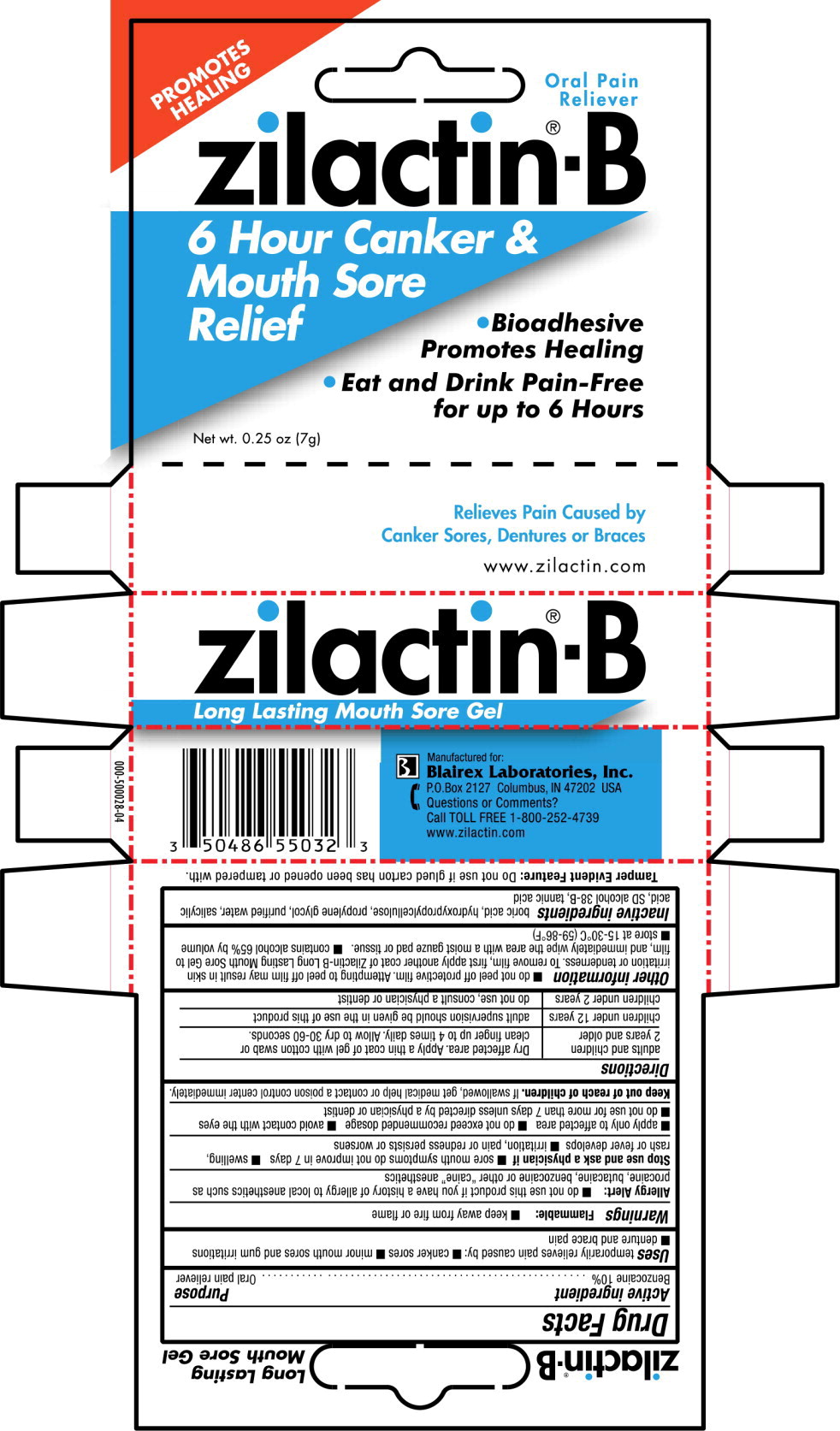
ZILACTIN-B- benzocaine gel
Applied Laboratories, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Zilactin B Applied
Use
temporarily relieves pain caused by:
- canker sores
- minor mouth sores and gum irritations
- denture and brace pain
Warnings
Allergy Alert:
- do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other “caine” anesthetics
Stop use and ask a physician if
- sore mouth symptoms do not improve in 7 days
- swelling, rash or fever develops
- irritation, pain or redness persists or worsens
- apply only to affected area
- do not exceed recommended dosage
- avoid contact with the eyes
- do not use for more than 7 days unless directed by a physician or dentist
Directions
| adults and children
2 years and older | Dry affected area. Apply a thin coat of gel with cotton swab or clean finger up to 4 times daily. Allow to dry 30-60 seconds. |
| children under 12 years | adult supervision should be given in the use of this product |
| children under 2 years | do not use, consult a physician or dentist |
Other information
- do not peel off protective film. Attempting to peel off film may result in skin irritation or tenderness. To remove film, first apply another coat of Zilactin-B Long Lasting Mouth Sore Gel to film, and immediately wipe the area with a moist gauze pad or tissue.
- contains alcohol 65% by volume
- store at 15-30°C (59-86°F)
| ZILACTIN-B
benzocaine gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Applied Laboratories, Inc. (117337220) |
Revised: 12/2021
Document Id: d21c544c-39da-0bb8-e053-2995a90ac6a2
Set id: e0ebcdbb-a75a-4a10-8686-457484fabe2d
Version: 4
Effective Time: 20211201
 Questions or Comments?
Questions or Comments?